April 30, 2025 feature
This article has been reviewed according to Science X's and . have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Molecular engineering approach could boost hydrogen evolution reaction activity by up to 50 times in alkaline media

Electrolyzers are devices that can split water into hydrogen and oxygen using electricity and via a process known as electrolysis. In the future, these devices could help to produce hydrogen gas from water, which is valuable for a wide range of applications and could also be used to power fuel cells and decarbonize energy systems.
At the core of the water electrolysis process are electrochemical reactions known as hydrogen evolution reactions (HERs). In basic (i.e., alkaline) conditions, these reactions tend to be slow, which in turn hinders the performance of electrolyzers.
In recent years, energy researchers have been trying to design new electrode-aqueous interfaces or identify new catalysts that could speed up HERs and thus enhance the ability of electrolyzers to produce hydrogen. One of the HER catalysts most employed to date is platinum, yet its performance is limited by a process known as hydrogen binding. This process entails the strong adherence of hydrogen atoms to its surface, which can block reaction sites and slow down HERs.
Researchers at Peking University, the Beijing National Laboratory for Molecular Sciences and other institutes in China recently introduced a new molecular engineering strategy that was found to speed up HERs on platinum electrocatalysts. This strategy, outlined in a paper in Nature Energy, entails the introduction of organic overlayers (i.e., thin molecular coatings that attach to the surface of electrodes).
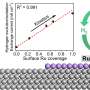
"Modifications at the surface of electrodes have been employed to accelerate HER, but effective guiding principles are lacking," wrote Kaiyue Zhao, Ningyao Xiang, and their colleagues in their paper. "We establish a molecular design strategy to enhance HER activity in alkaline media by up to 50 times by introducing an organic overlayer on Pt electrodes."
The researchers' newly proposed molecular engineering strategy modulates interactions on the surface of platinum catalysts, thus weakening the binding of hydrogen atoms and speeding up HERs. To test its potential and effects, the team carried out a series of tests, while also applying it to real electrolyzers with membrane electrode assembly (MEA) configurations.
"We find that enhancement of HER activity by organic adsorbates is correlated with their binding energies to Pt electrodes; binding energy could be tuned by changing the number of aromatic rings and hydrophilicity of the adsorbates," wrote Zhao, Xiang and their colleagues.
"Density functional theory calculations suggest that the overlayer led to a decrease in the d-band center, resulting in weakened H adsorption, which mitigated its overbinding on Pt. Importantly, we demonstrate the enhancing effect of the 2,2′-bipyrimidine overlayer on Pt/C in a water electrolyzer with a membrane electrode assembly configuration, confirming its effectiveness on the device level."
In initial experiments, the strategy devised by this team of researchers was found to weaken hydrogen absorption in electrolyzers with platinum catalysts, speeding up the rate of HER. In the future, their proposed strategy could also be applied to other catalysts beyond platinum, potentially contributing to the advancement of electrolyzers and facilitating their large-scale deployment.
More information: Kaiyue Zhao et al, A molecular design strategy to enhance hydrogen evolution on platinum electrocatalysts, Nature Energy (2025).
Journal information: Nature Energy
© 2025 Science X Network