A new electron microscopy technique reveals hydrogen storage processes in nanoscale

Sadie Harley
scientific editor

Robert Egan
associate editor

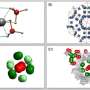
A research team from National Taiwan University has developed a new electron microscopy technique that enables sensitive atomic number (Z) measurements of samples. The technique, named atomic number electron microscopy (ZEM), is now used to observe hydrogen storage behavior and the associated defect formation and healing processes of palladium at the nanoscale.
This innovation may offer significant improvements for current material characterization tools, especially for quantitatively analyzing light elements or compounds.
Published in , the ZEM takes advantage of its sensitive responses to low Z materials, such as hydrogen (Z = 1) and vacancy defects (Z = 0). These features, which are nearly invisible under traditional electron microscopes, can now be visualized with remarkable clarity using ZEM.
"Light elements interact only weakly with electrons and photons, making them very difficult to detect," explains Dr. Chih-Wei Chang, the principal investigator of the paper. "But with our ZEM platform, we can finally directly observe hydrogen behavior inside metals and even see how it alters the material itself."
The team used palladium (Pd), a widely used material for hydrogen storage, as their experimental subject. They found that hydrogen absorption is not uniform, but tends to concentrate along grain boundaries and internal defects.
Even more surprisingly, after multiple cycles of hydrogen charging and discharging, the number of defects actually decreased, suggesting that hydrogen may play a role in promoting self-healing within the material.

ZEM enables non-destructive, quantitative analysis of hydrogen content and defect density, which sets it apart from traditional techniques that destroy the sample during analysis.
"Other techniques may be sensitive, but they can't revisit the same region of a sample," says Dr. Chang. "ZEM allows us to track dynamic changes throughout the hydrogen cycling process, which is critical in hydrogen material studies."
The research revealed that during early cycles, hydrogen preferentially fills existing vacancies and voids. As the cycles progress, hydrogen transitions to forming stable metal hydrides.
"We observed two distinct hydrogen uptake behaviors, something that was very difficult to distinguish previously," the authors note.
This study not only sheds light on how hydrogen is stored but also reveals the causality between hydrogen uptake and defect formation. The research team is continuously working to improve ZEM's sensitivity and spatial resolution.
"We hope that ZEM may become an essential tool in uncovering the microscopic secrets of many light-element materials in the future," said Dr. Chih-Wei Chang.
More information: Yu-Cheng Chiu et al, Quantitatively Profiling the Evolution of Hydrogen Storage and Defect Healing Processes in Palladium at the Nanoscale, ACS Nano (2025).
Journal information: ACS Nano
Provided by National Taiwan University