Highly oxidized products from isoprene鈥攁tmospheric chemistry pathways could drive global aerosol formation

Gaby Clark
scientific editor

Robert Egan
associate editor

Highly sensitive detection methods allow ever deeper insights into complex chemical processes in the atmosphere: Researchers at the Leibniz Institute for Tropospheric Research (TROPOS) in Leipzig found a series of new product channels in a detailed product study on the oxidative degradation of isoprene in the gas phase, which allows a better mechanistic understanding of this important process for atmospheric chemistry. The results were in the journal Nature Communications.
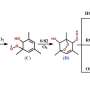
Isoprene (C5H8), mainly produced by deciduous forests, is one of the most important non-methane compounds with an annual emission rate of about 600 million metric tons of carbon released into the atmosphere. The degradation of isoprene takes place almost exclusively in the gas phase via reaction with the OH radical, which is regarded as an oxidative washing agent of the atmosphere. This initially produces structurally different HO-C5H8O2 peroxy radicals, which are in equilibrium with each other.
Despite their immense importance, their further reaction pathways are not yet fully understood. Isoprene reactions dominate the gas-phase processes, especially in the tropics, alongside those of methane (CH4) and carbon monoxide (CO).
The experiments at TROPOS were carried out in a large flow system at 1 bar air and room temperature. The reaction control was designed in such a way that product formation could be monitored for atmospheric concentrations of the reactive intermediates. Highly sensitive mass spectrometry was used to selectively detect the formation of peroxy radicals and stable products. The good agreement of the results using different ionization schemes confirmed the reliability of the findings.
The results of the investigations in the laboratory in Leipzig were largely in agreement with previous knowledge on the degradation of isoprene. However, some C4- and C5-products and their formation pathways were described for the first time. Of particular interest was the tracing of the formation of highly oxidized peroxy radicals of the composition C5H9O8 and C5H9O9, which are formed via so-called autoxidation processes on a short time scale of seconds.

"The molar yield of the highly oxidized species as a function of competing processes is only 0.3% at most, which seems negligible at first," says Dr. Torsten Berndt from TROPOS, who planned and carried out the experiments. "However, the huge emission rate of isoprene makes the formation of C5H9O8 and C5H9O9 potentially very interesting on an absolute scale."
In order to further assess the significance of these reaction pathways, they were then implemented in a global chemistry-climate model. The model simulations carried out showed that around 4 million metric tons of highly oxidized isoprene peroxy radicals (C5H9O8 and C5H9O9) are formed globally every year.
"Interestingly, the modeled production rates are of the same order of magnitude as the formation of the analogous products (C10H17O7 and C10H15O8,10) from the oxidation of 伪-pinene. This formation pathway was previously regarded as the most important process for the formation of highly oxidized gas-phase products in the atmosphere," add the scientists responsible for the global modeling, Dr. Erik Hoffmann and Dr. Andreas Tilgner.
In a further reaction step, the C5H9O8 and C5H9O9 radicals react in the atmosphere mainly with nitrogen monoxide (NO) and hydroperoxy radicals (HO2). The reaction with NO leads mainly to the formation of the corresponding highly oxidized organic nitrates if the chemical C5-structure is retained. The product formation in the case of the reaction with HO2 is still speculative and requires further investigation.
In conclusion, the scientists emphasize that the new research findings raise a number of questions. The highly oxidized products of isoprene could influence a variety of processes such as the growth of atmospheric aerosol particles and secondary organic matter formation.
These processes are closely linked to important aerosol-cloud interactions and their global climate and radiative effects. Therefore, considerably more studies are needed to adequately clarify the significance of the new oxidation pathways and the resulting products for the atmosphere.
More information: Torsten Berndt et al, Highly oxidized products from the atmospheric reaction of hydroxyl radicals with isoprene, Nature Communications (2025).
Journal information: Nature Communications
Provided by Leibniz-Institut f眉r Troposph盲renforschung e. V.