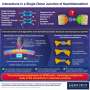
A novel hybrid charge transfer crystal with reversible color-changing property

Transfer of charge is a process in which electrons move within a molecule or between two molecules. It is a crucial chemical process that can be applied to a wide range of technologies. Intramolecular charge transfer (ICT) occurs when electrons are exchanged between donor and acceptor groups within a molecule via a series of overlapping electron orbits. This exchange creates a shift in light wavelength toward the red end of the light spectrum (redshift). This observable color shift due to ICT has applications in dye manufacturing and organic LEDs (OLEDs).
Intermolecular charge transfer (CT), when electrons are exchanged between different molecules, can be realized using "π-conjugated organic molecules" through which electron movement occurs from donors to acceptors. CT plays a crucial role in photovoltaic devices, semiconductors, and other applications.
Combining CT with ICT in a single hybrid system could lead to the development of novel materials. However, achieving this has been challenging because it requires precise control over the molecular design and intermolecular interactions. Additionally, the hybrid system must be composed of material that remains stable under such rapid transfer conditions.
Pyrazinacenes, a class of aromatic (ring-like) molecules, could be a promising candidate for such a task. Pyrazinacenes can serve as a bridge between a donor and acceptor molecule, facilitating CT. Being deficient in electrons, pyrazinacene enables electrons to move easily within its ring-like structure, facilitating ICT. This may lead to the formation of a CT-ICT hybrid system, though its efficacy remains untested.
Now, in a new study, scientists from the Graduate School of Engineering and Science, Shibaura Institute of Technology (SIT), Japan—Professor Akiko Hori, Mr. Kazushi Nakada, and Dr. Gary James Richards—describe a new CT-ICT system (compound 1) that utilizes the novel pyrazinacene derivative, 6,7-bis{4-(diphenylamino)-phenyl}-pyrazino[2,3-b]pyrazine-2,3-dicarbonitrile.
This pyrazinacene core links triphenylamine groups, strong electron donors, with cyano groups, electron acceptors. The study was in Chemistry—A European Journal on March 25, 2025.
Compound one co-crystallized in a 1:1 ratio with naphthalene. The resulting crystals exhibited a dramatic color change, shifting from greenish-blue to red-violet. This interaction was specific to naphthalene. Experiments with naphthalene derivatives, such as octafluoronaphthalene, did not result in co-crystallization; instead, they led to electronic repulsion.
Thermogravimetric analysis and powder X-ray diffraction confirmed compound 1's specificity for naphthalene and demonstrated successful co-crystallization. Density functional theory (DFT) calculations revealed that the distinct color shift arises from an intermolecular CT event facilitated by the novel pyrazinacene. The CT event disrupts ICT, resulting in a blue shift.
"Our molecule's design achieves competition between intramolecular and intermolecular charge transfer," says Nakada, a graduate student at SIT and the first author of this paper. Elaborating further, he says, "This allows our molecule to act as a sensor that can, through a simple color change, identify even trace amounts of naphthalene—an environmentally regulated substance—in freshwater and seawater."
Upon analyzing the crystal structure, the researchers found that the molecular recognition process, responsible for the color shift in these crystals, is facilitated by π-hole···π interactions. The hydrogen atoms of naphthalene are extended toward the nitrogen atoms of the pyrazinacene (compound 1); however, the atoms are not close enough to form strong hydrogen bonds. Instead, the crystal structure is stabilized by weaker Van der Waals forces.
These weaker bonds can be easily broken and reformed, making the color shift a reversible process. For instance, heating the violet crystals to 180 °C caused naphthalene to separate, restoring the crystals to their original greenish-blue color.
"Our study establishes a foundation for synthesizing nonporous adaptive crystals with reversible color-changing properties. This breakthrough opens new avenues for the development of sensor technologies and materials for selective molecular recognition," concludes Prof. Hori.
More information: Kazushi Nakada et al, Colorimetric Detection of Naphthalene Enabled by Intra‐ to Intermolecular Charge Transfer Interplay Induced by π‐hole⋅⋅⋅π Interactions of a TPA‐Attached Pyrazinacene, Chemistry – A European Journal (2025).
Journal information: Chemistry – A European Journal
Provided by Shibaura Institute of Technology