Mass spectrometry method for analyzing surface structure of lipid nanoparticles could improve vaccine and drug delivery

Sadie Harley
scientific editor

Robert Egan
associate editor

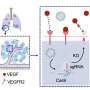
A team led by scientists at the University of Nottingham's School of Pharmacy demonstrated a new cryogenic mass spectrometry approach for depth profiling frozen tiny lipid nanoparticles to reveal the layers and orientation of the constituent molecules.
The findings have been published in
Lipid nanoparticles (LNPs) came to prominence for the delivery of RNA with the success of the Moderna and Pfizer BioNTech COVID-19 vaccines. They are also used to deliver therapeutic treatments, including using small interfering RNA-based drugs to treat the rare hereditary disease of polyneuropathy (Alnylam Pharmaceuticals).
Areas of development include lung-targeted gene therapy, which is particularly challenging, but LPNs have the potential to treat a range of diseases including cystic fibrosis, idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, asthma and more.
This research provides insight into the relative positions of each component within lipid nanoparticles. This knowledge can help clarify the intricate behavior of LNPs and contribute to the design of formulations with unique bio-properties that are more efficient and safer.
The research findings could also be used in the future to help quality control during the scale-up of manufacturing processes, enhancing the translation of LNPs from the laboratory to clinical applications.
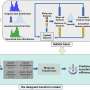
The research team, which also included Sail Biomedicines, Cambridge, MA, Massachusetts Institute of Technology, Cambridge, MA and the National Â鶹ÒùÔºical Laboratory, Teddington, UK, utilized Cryogenic Orbitrap secondary ion microscopy (Cryo-OrbiSIMS) to provide structural details of the lipid nanoparticles. This high-pressure-freezing cryo-preparation facility keeps biological samples maintained close to their native state.
Professor Morgan Alexander, who led this research, explains, "Characterizing the native surface of delicate hydrated pharmaceutical systems used in the body has been a significant challenge for some time. This cryogenic molecular surface and interfacial analysis advance makes this exciting possibility real.
"We expect to apply this new method to many systems, including lipid nanoparticles, other pharmaceutical delivery systems and hydrated biomaterials."
Dr. Robert Langer, from Massachusetts Institute of Technology, is also an author of the research paper. He said, "Effective drug delivery relies on an intricate mix of molecules in lipid nanoparticles to effectively deliver RNA therapeutics, but these can vary in efficacy and can be difficult to engineer.
"This research provides a new way of characterizing and understanding the make-up of lipid nanoparticles which could pave the way for engineering more potent and targeted LNPs to enable the broadest application of RNA therapies for all types of diseases."
"At Sail Biomedicines, we are proud to have contributed to advancing the understanding of lipid nanoparticle surface structures," said Kerry Benenato, Ph.D., Chief Platform Officer at Sail Biomedicines.
"The surface of lipid nanoparticles plays a critical role in shaping their behavior in the human body. By enabling precise surface characterization, the technology the team has developed paves the way for the engineering by design of LNP-based medicines with tunable properties, including biodistribution, thereby expanding the potential of RNA-based therapeutics."
More information: Anna M. Kotowska et al, Study on molecular orientation and stratification in RNA-lipid nanoparticles by cryogenic orbitrap secondary ion mass spectrometry, Communications Chemistry (2025).
Journal information: Communications Chemistry
Provided by University of Nottingham