Reexamining a receptor linked to sepsis resolves contradictions regarding its binding interactions

After earning notoriety as the first cellular receptor isolated and mammalian lectin identified, the Ashwell-Morell receptor's functions in our bodies eluded scientists for more than 30 years.
In 2008, scientists in San Diego shed new light on this landmark liver cell receptor by identifying its roles in sepsis. Those experiments revealed that the Ashwell-Morell receptor's binding partners—known as ligands—modulated blood clotting in determining host survival of sepsis.
"It's an important receptor for a number of functions, including control of inflammation and coagulation," said Jamey Marth, Ph.D., a professor at Sanford Burnham Prebys. "And it is a critical player during sepsis, a condition that kills more people than cancer each year. Our study of this receptor impressed upon me how there's no faster way to alter the levels and thus functions of components in the bloodstream than to change their rates of clearance."
As researchers continued to investigate the Ashwell-Morell receptor, however, conflicting reports emerged regarding the characteristics of the receptor's ligands in the bloodstream. To better understand how the receptor maintains normal levels of proteins and components in the bloodstream, they needed to know which ligands are relevant. Inconsistent results in published studies made this more challenging.
Marth, his team at Sanford Burnham Prebys, and colleagues at the University of Copenhagen and Leiden University Medical Center have their findings in Proceedings of the National Academy of Sciences with the goal of determining the composition and topology of physiological Ashwell-Morell receptor ligands. Their findings will help uncover the receptor's still-hidden secrets.
Marth recognized that technological limitations contributed to the field's incongruous findings, so his team took a new approach in the recently published study. To overcome these technical hurdles, the researchers used an innovative technique called glycoengineering.
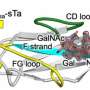
Chains of sugars, termed glycans, are found modifying most secreted proteins and can play many roles in addition to receptor ligands. The scientists edited the genes of cells controlling glycan formation to coax them into producing near-homogeneous and discrete glycan structures on different protein glycoforms.
Production and study of these glycoforms on intestinal alkaline phosphatase (IAP), a known Ashwell-Morell receptor ligand, made all the difference. It allowed Marth and his team to clarify the effect of each of the glycan linkages on binding to the Ashwell-Morell receptor.
"Without the ability to engineer near-homogeneous glycoforms of proteins, it was just not possible to do this experiment properly and reach a definitive conclusion," said Marth, the senior and corresponding author of the study.
The team's results were consistent with an absence of Ashwell-Morell receptor ligands on glycoforms of IAP featuring glycan chains with two antennae, known as biantennary. This severely restricts the receptor's potential pool of ligands, given that most proteins circulating in the bloodstream are modified by biantennary glycan chains. Yet, IAP is a ligand.
"We found that when IAP dimerizes, two biantennary sugar chain structures are brought into close proximity, thereby mimicking multi-valent tri- and tetra-antennary glycan structures required for Ashwell Morell receptor binding," said John Hintze, Ph.D., a postdoctoral associate in the Marth lab and first author of the study.
"By protein folding and oligomeric assembly, the more common biantennary sugar chains can overlap to create feasible ligands, explaining how the Ashwell-Morell receptor binds and clears more than a third of all proteins in blood plasma."
In addition to their work on the effects of these different sugar chain decorations, Marth and his collaborators clarified contradictory findings regarding sialylation, a modification that occurs when sialic acid is added to the end of the glycan chains.
"There have been multiple studies in the last 10 years or more that suggested the Ashwell-Morell receptor could detect and clear ligands with select sialylated structures, but that was at odds with other published results," said Marth.
Marth's team found that the receptor could only bind IAP if the sialic acid modification had been removed, and presented various explanations for previous disparate results.
Marth intends to continue studying the Ashwell-Morell receptor and other similar cellular receptors to learn more about how they survey the bloodstream and control the blood proteome.
"It is important to advance our understanding of what controls the normal abundance and thus function of blood proteins in circulation," said Marth. "Excursions from normality are among the diagnostic criteria physicians use to detect and prognose disease."
More information: John Hintze et al, Compositional and topological determinants of a physiological Ashwell–Morell receptor ligand, Proceedings of the National Academy of Sciences (2025).
Journal information: Proceedings of the National Academy of Sciences
Provided by Sanford-Burnham Prebys