Chemists develop new method to synthesize chiral bridged polycyclic compounds for drug discovery

Gaby Clark
scientific editor

Robert Egan
associate editor
![A schematic of the catalytic enantioselective type II [5 + 2] cycloaddition method. Credit: HKUST HKUST chemists innovate in the synthesis of chiral bridged polycyclic compounds](https://scx1.b-cdn.net/csz/news/800a/2025/hkust-chemists-innovat.jpg)
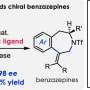
A Hong Kong University of Science and Technology (HKUST) research team has developed a catalytic enantioselective type II [5 + 2] cycloaddition method to address the challenges of synthesizing chiral bridged polycyclic structures, particularly those with a bridged seven-membered subunit.
This innovative approach utilizes 3-oxidopyrylium ylides to create the desired complex shapes, paving the way for more applications in the rapid synthesis and diversification of other valuable complex molecules, including important natural products and drug molecules.
The study is in the journal Nature Synthesis. The team was led by Prof. Sun Jianwei and Prof. Lin Zhenyang from the Department of Chemistry.
Chiral bridged polycyclic structures, particularly those bearing a bridged seven-membered subunit, represent a complex and intriguing molecular architecture found in many natural and biologically significant compounds. However, synthesizing these structures has posed substantial challenges for chemists.
Among the available methods, the intramolecular [5+2] cycloaddition of versatile dipolar molecules like 3-oxidopyrylium ylides stands out as one of the few efficient processes for accessing such molecular complexity. Notably, this research marks the first report of a catalytic enantioselective type II cycloaddition, a significant advancement given its potential for synthesizing diverse complex natural products.
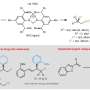
The research team successfully addressed the formidable challenge of preventing the formation of strained, anti-Bredt cycloadducts bearing bridge-head double bonds while exerting enantiocontrol. They achieved this through non-covalent activation by chiral acid catalysis, a novel approach compared to the traditional covalent activation methods.
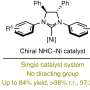
Remarkably, chiral phosphoric acids (CPAs) derived from the SPHENOL backbone, developed in their own laboratories, proved to be particularly effective in inducing enantioselectivity. The chiral acid catalyst not only promoted the rate-determining enolization step but also provided the necessary asymmetric induction for the formation of enantioselective C–C bonds, showcasing a dual functionality that enhances the overall efficiency of the cycloaddition process.
The protocol provides various functionalized and bridged carbocycles, which can serve as advanced intermediates in the synthesis of complex molecules. Some of these carbocycles are already core structures of important natural products and drug molecules.
"Additionally, this protocol is expected to be applicable to other cycloadditions and would find more applications in the rapid synthesis and diversification of other useful complex molecules," Prof. Sun said.
More information: Liangliang Yang et al, Enantioselective type II intramolecular [5 + 2] cycloadditions of oxidopyrylium ylides using chiral-phosphoric-acid catalysis, Nature Synthesis (2025).
Journal information: Nature Synthesis
Provided by Hong Kong University of Science and Technology