Chimera approach overcomes mitochondrial barrier to alter protein production in living cells

Sadie Harley
scientific editor

Robert Egan
associate editor

Mitochondria supply the body with the energy currency adenosine triphosphate (ATP), which drives all bodily activities. For ATP production, the mitochondria consume around 95% of the oxygen inhaled. This process takes place in the so-called respiratory chain in the mitochondria, which is built of numerous individual proteins.
Malfunctions in the respiratory chain lead to serious and often fatal diseases that can affect skeletal muscle and nerve cells, as well as the heart.
Mitochondria have their own genetic material, mitochondrial DNA, or mtDNA for short. This is required for the production of 13 central proteins of the respiratory chain. Many of the mitochondrial hereditary diseases are caused by defects in the mtDNA, which lead to a loss of function of the respiratory chain and thus to a disruption of the cell's energy supply.
How the mtDNA is read and translated into proteins has not yet been sufficiently clarified. The reason for this is that there are currently no techniques available to influence protein production in mitochondria.
Researchers led by Prof. Dr. Peter Rehling, director of the Department of Cellular Biochemistry at the University Medical Center Göttingen (UMG) and member of the Göttingen Cluster of Excellence "Multiscale Bioimaging: From Molecular Machines to Networks of Excitable Cells" (MBExC), are now one step further: they have succeeded in developing a new technology that can be used to alter protein production in the mitochondria of living cells.
The results of the study are in Science. The knowledge gained with the help of this new technology makes it possible to understand the fundamental principles of cellular energy production and thus also to draw conclusions about the development of mitochondrial diseases.
The study in detail
The process of producing key respiratory chain proteins in the mitochondria is very complex. Defects in the process can lead to a variety of disorders, including cardiovascular disorders and disorders of the nervous system. "In order to understand the molecular mechanisms of protein production in mitochondria, we need experimental approaches that enable us to influence the individual steps of the process," says Prof. Rehling, senior author of the publication.
"Such technologies help us to understand how disturbances of a biological process challenge the cells. We can also use them to investigate how the cells react to such challenges in order to compensate for them." Until now, however, experimental strategies for investigating protein production in mitochondria have been lacking.
Established techniques, such as CRISPR, which can be used to specifically alter genetic material, do not work in mitochondria, as the mitochondrial membrane represents an insurmountable barrier for the gene scissors.
Targeted deactivation of proteins in living cells
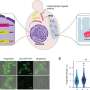
The new technique developed by researchers in Göttingen overcomes the mitochondrial barrier. They use a chemically modified small protein fragment known as a chimera. It contains the necessary information, a kind of "zip code," to enter the mitochondria. Once there, it interferes specifically in the process of protein production.
In order for a protein to be made, a copy of the genetic material, in this case the mtDNA, must first be created. This copy contains the blueprint of the protein to be produced. The chimera was constructed in advance in such a way that it specifically attaches itself to the blueprint of a selected protein, thereby blocking the further steps in the production of a functional protein. In this way, it is possible to investigate how the cell's metabolism changes when certain proteins are no longer produced.
The researchers were also able to influence protein production in the mitochondria of heart muscle and liver cells. "With the help of the new technology, it is now possible for the first time to investigate how cells react to very specific disturbances in protein production," says Dr. Luis Daniel Cruz-Zaragoza, group leader at the Institute of Cell Biochemistry at the UMG and first author of the study.
"This opens up completely new possibilities for gaining insights into the development of mitochondrial diseases and developing new therapies on this basis."
More information: Luis Daniel Cruz-Zaragoza et al, Silencing mitochondrial gene expression in living cells, Science (2025).
Journal information: Science
Provided by Universitätsmedizin Göttingen