Novel electroenzymatic strategy enables non-natural oxidation reactions

Sadie Harley
scientific editor

Robert Egan
associate editor

A research team led by Prof. Xiaoqiang Huang from Nanjing University has developed a novel non-natural dynamic kinetic oxidation system by integrating ferrocene methanol-mediated anodic oxidation with thiamine diphosphate (ThDP)-dependent enzyme catalysis, marking a significant advance in the field of asymmetric electroenzymatic catalysis.
The work, supported by electron paramagnetic resonance (EPR) measurements at the Steady-State Strong Magnetic Field Facility of the Hefei Institutes of Â鶹ÒùÔºical Science of the Chinese Academy of Sciences, is published in .
Electrochemistry is undergoing a resurgence in synthetic chemistry due to its sustainability and unique activation modes. While natural enzymes have been repurposed through tools like directed evolution and photoenzymatic catalysis, integrating enzymes with electricity to achieve new-to-nature reactivity has long been limited by compatibility issues and inefficient electron transfer.
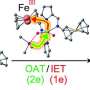
To overcome these challenges, Prof. Huang's team ingeniously combined ferrocene methanol as an electron mediator with ThDP-dependent enzymes. By engineering the enzyme's active site via directed evolution and introducing a two-step single-electron oxidation mechanism, they successfully unlocked a non-natural, electricity-driven enzymatic transformation.
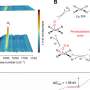
Mechanistic studies, including cyclic voltammetry and low-temperature EPR, confirmed that the mediator efficiently enabled single-electron transfer between the electrode and the enzyme's Breslow intermediate. The corresponding free radical intermediates were clearly detected via EPR.
Using this electroenzymatic platform, the team achieved efficient oxidation of α-branched aldehydes, enabling the synthesis of a variety of (S)-profens—key intermediates in anti-inflammatory drugs—with up to 99% enantiomeric excess. The process proved effective at enzyme loadings as low as 0.05 mol% and could be applied using whole-cell biocatalysts.
Further mechanistic insights revealed that the engineered electroenzyme plays multiple roles: it precisely recognizes substrates, accelerates racemization, and ensures kinetically synchronized electron transfer, making the system highly efficient and selective.
This study opens a new chapter in biocatalysis, providing a robust electroenzymatic approach for developing non-natural transformations and expanding the toolbox for asymmetric synthesis in green chemistry.
More information: Beibei Zhao et al, Electricity-driven enzymatic dynamic kinetic oxidation, Nature (2025).
Journal information: Nature
Provided by Chinese Academy of Sciences