Eliminating external catalysts for the sustainable synthesis of biomolecules and pharmaceuticals

Lisa Lock
scientific editor

Robert Egan
associate editor

In the future, it may be possible to produce bioactive molecules and pharmaceuticals without reverting to using enzymes or metals as external catalysts. Chemists at Friedrich-Alexander-Universit├Ąt Erlangen-N├╝rnberg (FAU) have developed a procedure during which an in situ-formed organoautocatalyst allows for extremely effective chemical synthesis of bioactive cyclical amine compounds under mild conditions. The findings are in the journal Angewandte Chemie International Edition.
The synthesis of cyclic aminesÔÇöring-shaped molecules based on nitrogen and carbonÔÇöis increasingly gaining significance in medicine and biochemistry. Dihydropyridine is of particular interest, a six-membered ring with five carbon atoms and one nitrogen atom. Dihydropyridine compounds are used, for instance, to lower blood pressure, and due to their adjustable fluorescence are also being considered for use as photoelectronic materials.
"A number of different molecules can bind to the nitrogen atom. This variation in substituents allows researchers to take a targeted approach to modulating the biological properties of dihydropyridines," explains Prof. Dr. Svetlana Tsogoeva, head of the working group at the Department of Chemistry and Pharmacy at FAU.
Until now, synthesis has proven complicated, expensive and often toxic
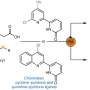
Transamination reactions, in other words, the targeted variation of the substituent on the nitrogen atom in cyclic and acyclic amines, pose a great challenge for synthetic chemistry. "At the current time, complex enzyme catalysts or expensive and often toxic metals are required, and the reactions usually take place under extreme conditions," explains Tsogoeva. This not only makes synthesis difficult and cost-intensive, it also leads to toxic waste, which is particularly problematic when it comes to the production of medicines.
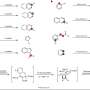
The Tsogoeva group has now proposed a procedure that completely refrains from using external catalysts and yet is still highly effective. The researchers use pyrrolidinium salt, an ammonium salt that is formed during the synthesis process and accelerates the reaction, as an organoautocatalyst. The impressive result: the autocatalyst reaction takes place in a singular domino-like process at room temperature and provides a yield of up to 95%.
Tsogoeva states, "This procedure goes above and beyond imitating nature and opens new possibilities in the chemistry of carbon-nitrogen compounds."
The new organoautocatalyst system establishes an efficient, sustainable strategy for easily accessing complex bioactive molecules and pharmaceutical compounds containing nitrogen. The study not only contributes to gaining a basic understanding of the substitution of different molecule groups in carbon-nitrogen compounds. It also opens fascinating possibilities for developing green synthesis methods of the next generation without using enzymes, metals or aggressive reagents.
More information: Volker Klein et al, Development of an Organoautocatalyzed Double ¤âÔÇÉBond C(sp2)ÔÇÉN Transamination Metathesis Reaction, Angewandte Chemie International Edition (2025).
Journal information: Angewandte Chemie International Edition