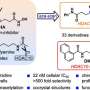
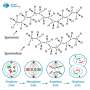
Model of the modes of PUT acquisition, polyamine biosynthesis, and eIF5A hypusination used by B. duncani and P. falciparum. Credit: Science Advances (2025). DOI: 10.1126/sciadv.adv2397
From cancer to infectious diseases to neurodegenerative diseases such as Alzheimer's disease and Parkinson's disease, there is an undeniable need for new treatments and medications.
And while some researchers are racing to find drugs that can tackle everything from the mysterious mechanisms of these diseases to rapidly developing antimicrobial resistance, drug discovery can be an arduous and slow process—one almost akin to finding a needle in a haystack.
Researchers may screen millions of potential compounds before finding one that works, and even then, that compound must successfully make its way through multiple levels of biomedical and clinical testing before becoming a viable treatment.
Now, researchers at Yale School of Medicine have uncovered a critical role for a group of molecules called polyamines in parasite development. These findings, June 18 in Science Advances, then led the team to develop a first-of-its-kind assay that has the potential to enhance drug discovery for a wide variety of diseases, including cancer and neurodegeneration.
The research was led by Choukri Ben Mamoun, Ph.D., John F. Enders Professor of Medicine (Infectious Diseases), and the new assay was described in the and . Pallavi Singh, Ph.D., and Jae-Yeon Choi, Ph.D.—senior scientists in the Ben Mamoun lab—conducted the research.
Here, Ben Mamoun, who is also a professor of pathology and of microbial pathogenesis, discusses how his research on parasites and polyamines led to the development of this assay and what it means for drug discovery. This interview has been edited for clarity and length.
Choukri Ben Mamoun discusses how his parasite research led to a new tool for drug discovery that has applications for infectious diseases, cancer, and neurodegeneration. Credit: Yale University
You're a parasite researcher by trade, and this all started with sort of an accidental discovery while you were growing Babesia duncani in the lab, a parasite that causes the tick-borne disease babesiosis. What happened?
It was pure serendipity during the COVID-19 pandemic. We ran out of the culture media we used to grow our parasites, and the companies that supplied the media would not disclose the formulation. To solve this problem, we tried to recreate the media ourselves and found that the parasites could grow in one version but not in another.
The major difference between the two versions we had made was that one contained additional nutrients, including a polyamine called putrescine. And that's how we discovered that putrescine is essential for parasite growth.
What exactly are polyamines?
These are unique molecules, and there are three: putrescine, spermidine, and spermine. Polyamines are found in nearly all living cells, but their precise functions have not been well-defined. What we do know is that they help protect cells from potentially damaging reactive molecules, and they also play a role in stabilizing DNA and RNA.
While it has been known that polyamines are essential for life, it wasn't clear which one plays the most critical role in parasite development and survival. One of the key findings in our Science Advances paper is that spermidine is the key polyamine. That was not really known before. Spermidine is converted into a unique molecule called hypusine, which is essential for protein translation, which is essential for life, and, therefore, crucial for parasite development and survival.
So overall, polyamines carry out multiple essential functions in cells. These roles have been implicated not only in infectious diseases but also in cancer and neurodegenerative disorders. That's what makes our findings particularly exciting; because polyamines are so broadly important, they offer a potential target for a wide range of diseases.
So if we can find drugs that stop polyamine synthesis, we stop the growth of parasites, bacteria, fungi, and even cancer cells. How exactly do we find those drugs?
For a long time, chemists have developed compounds that simply look like polyamines, which basically get in the way and inhibit polyamine synthesis. But direct inhibitors of the enzymes that produce polyamines have not been developed, largely because there wasn't a practical way to test millions of molecules at once, which is what you'd really need to do to identify an effective inhibitor.
And that's been the major hurdle in the field: Without an assay that's amenable to high-throughput screening, it's nearly impossible to discover new direct inhibitors. It's not that it can't be done; it's just extremely difficult. That challenge is what led us to develop an entirely new assay.
We had already shown that the enzymes responsible for polyamine synthesis, especially the one that produces spermidine, are essential. So we asked: How can we design an assay to screen chemical libraries for inhibitors of these enzymes?
What we created is a fluorescence-based assay that uses a small molecule capable of binding polyamines and producing distinct fluorescence signals depending on which polyamine is present. This allows us to measure whether putrescine, spermidine, or spermine remain after adding potential enzyme inhibitors.
With this approach, we can now screen large chemical libraries, containing millions of compounds, to find molecules that can inhibit specific polyamine synthesis enzymes. These enzymes are relevant not only in infectious diseases but also in cancer, neurodegenerative disorders, and other conditions. And that's exactly what we are focused on now.
Polyamines are essential for all cells, which means that our healthy cells need them, too. How do we ensure that these inhibitors only stop polyamine synthesis in pathogenic cells, and not healthy ones?
If the goal is to inhibit the enzymes in pathogens, we focus on the differences between the pathogen's enzyme and the human counterpart. These enzymes are often different enough that we can specifically target the pathogen's version without affecting the human one. So, we design and screen for inhibitors that selectively block the pathogen enzyme while leaving the human enzyme untouched.
In the case of cancer, we are targeting human cells, but the distinction lies between normal and cancerous cells. Cancer cells tend to have much higher levels of polyamines and polyamine-synthesizing enzymes. We take advantage of this difference by identifying drug doses that inhibit cancer cells while sparing normal ones. It's a matter of exploiting the cancer cell's dependency on elevated polyamine production.
Did you ever expect that what started as a study on these Babesia parasites you've been studying for a decade would turn into such a powerful drug discovery tool?
I think this research highlights something unique, which is that our studies of Babesia can actually inform studies on a broad range of pathogens. One of the major advantages of the Babesia species employed in our Science Advances paper, Babesia duncani, is that we can culture it in vitro and propagate it in mice. This dual capability makes it an especially powerful model for both discovery and preclinical testing.
Another important point is that the enzymes we're studying are conserved across many pathogens. As a result, the inhibitors we identify could end up being what we call pan-antimicrobial agents—drugs that act against multiple pathogens.
That means if we invest in developing a drug to target Babesia, that same compound could potentially be applied to other parasites such as Plasmodium, the causative agent of malaria, and Leishmania and Trypanosomes species, the causative agents of leishmaniasis and trypanosomiasis, respectively.
These inhibitors could also have applications against fungal infections, including multidrug-resistant pathogens like Candida auris. It is a promising and efficient strategy for achieving broader therapeutic impact.
More information: Pallavi Singh et al, Spermidine is a key polyamine required by intracellular parasites for survival within host erythrocytes, Science Advances (2025).
Pallavi Singh et al, A fluorescence-based assay for measuring polyamine biosynthesis aminopropyl transferase–mediated catalysis, Journal of Biological Chemistry (2024).
Pallavi Singh et al, A fluorescence-based assay for measuring aminopropyltransferase activity, Enzymes of Polyamine Metabolism (2025).
Journal information: Journal of Biological Chemistry , Science Advances
Provided by Yale University