A smarter way to search for antibiotics: Focus on the genetic switches

Lisa Lock
scientific editor

Robert Egan
associate editor

Bacteria carry countless hidden treasures in their DNA—fragments that could hold the key to new medicines. But how do you pick out the most promising ones from millions of options? "Look at the switches that turn genes on and off," says molecular biologist Gilles van Wezel.
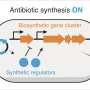
Van Wezel and his team have developed a new and more effective way to search for antibiotics. They focus on when certain bits of DNA are switched on or off. If you can identify the "switch" that controls a fragment, you can also predict what that bit of DNA does—for instance, whether it plays a role in producing an antibiotic.
There are still millions of gene clusters in bacteria such as Streptomyces whose potential remains unknown. With this new method, the team hopes to uncover the most valuable candidates more quickly.
'We've rewritten the production process of a known molecule'
To test their method, Van Wezel's team took a closer look at a genetic switch called DmdR1. This switch helps Streptomyces bacteria absorb iron and also controls the production of desferrioxamine (DFO), a molecule that binds iron. Scientists thought they already understood exactly how DFO was made—but that turned out not to be the case.
The researchers discovered a previously unknown group of genes that DmdR1 also switched on and off. Further analysis showed that these genes are also essential for making DFO. "We've effectively rewritten the production process of a known molecule," Van Wezel says. The findings are in the journal PLOS Biology.
Searching for similar switches
For the first time, a new function has been linked to a group of genes by studying how they're switched on and off. The concept works and these results pave the way for new opportunities. "Once you understand how a switch functions, you can scan other DNA for gene clusters that use the same one," Van Wezel explains.
"Take a switch that's activated when a bacterium produces antibiotics during an infection. You don't need to know exactly which molecule is involved or what the genes do. If the system responds to the same signal in the same way, there's a good chance the molecule it produces has a similar function."
To speed up the search for these genetic "hidden treasures," Van Wezel also wants to use artificial intelligence. "Can we predict the biological activity of a molecule just by looking at its DNA?" That would allow researchers to narrow down their search—from millions of potential candidates to just a few thousand or even a hundred. "That would make it far more realistic to study each one in detail."
Mapping the genetic switches
The biggest challenge? Figuring out exactly where in the DNA these molecular switches bind. A lot of that work is being done by Ph.D. student Hannah Augustijn, in collaboration with Marnix Medema (IBL and Wageningen University). Together with the Joint Genome Institute in Berkeley, California, they are mapping out these binding sites.
Until recently, scientists only knew the binding location for about 3% of the switches in Streptomyces bacteria. This team is now pushing that number up to 30% or even 40%. "We now know which genes are controlled by hundreds of transcription factors—the proteins that turn genes on or off. That helps us much more accurately predict which gene clusters are worth investigating further."
A new 'golden age' of discovery may be getting closer
This method can be applied broadly, says Van Wezel—from gut bacteria and disease-causing microbes to microorganisms found on plants. "We're sharing all our findings with the scientific community, so others can build on our work.
"We want to offer a useful tool to anyone searching for new bioactive compounds—whether that's antibiotics, anti-cancer agents or treatments for plant diseases." It seems the long-awaited "golden age" of discovery is finally within reach.
More information: Hannah E. Augustijn et al, Genome mining based on transcriptional regulatory networks uncovers a novel locus involved in desferrioxamine biosynthesis, PLOS Biology (2025).
Journal information: PLoS Biology
Provided by Leiden University