Nano-engineered photocatalyst sets milestone for solar fuel production

Lisa Lock
scientific editor

Robert Egan
associate editor

In a leap forward for solar fuel technology, researchers from Japan have developed nanosized, porous oxyhalide photocatalysts (Pb2Ti2O5.4F1.2) that achieved record performance in producing hydrogen from water and converting carbon dioxide to formic acid using sunlight, outperforming previous oxyhalide catalysts by ~60 times. This breakthrough offers a scalable, eco-friendly approach to solar fuel production and highlights the importance of controlling particle size and structure to enhance efficiency.
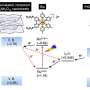
In a shift towards clean energy, scientists are working to harness sunlight not only for electricity but also to produce useful fuels. This transformation is made possible by photocatalysts—materials that can absorb visible light and trigger chemical reactions. When exposed to light, these catalysts generate charge carriers (electrons and holes) that drive reactions such as splitting water into hydrogen (H2) and converting carbon dioxide (CO2) into formic acid, a liquid fuel and hydrogen carrier.
Among the most promising photocatalysts for these applications are lead-based oxyhalides Pb2Ti2O5.4F1.2 (PTOF). These materials have a narrow bandgap, which enables efficient absorption of visible light, and can resist harsh oxidative conditions, which are key properties for long-term catalytic performance.
In a breakthrough, researchers from Institute of Science Tokyo (Science Tokyo), Japan, have synthesized smaller, more porous nanosized PTOF particles, achieving photocatalytic activity up to ~60 times higher than previously reported. The study—led by Professor Kazuhiko Maeda from the Department of Chemistry, School of Science, Science Tokyo, and Professor Osamu Ishitani from Hiroshima University, Japan—is in ACS Catalysis.
"The synthesis method established in this study enables world-leading photocatalytic performance for H2 production and the conversion of CO2 into formic acid among oxyhalide photocatalysts, using an environmentally friendly process," says Maeda.
Reducing the particle size enhances catalytic activity by shortening the distance that photogenerated charge carriers must travel to reach the surface, lowering the likelihood of their recombination. However, it can also introduce structural defects, which may negatively impact performance. In this study, the researchers show that their method can avoid these problems by carefully controlling the shape and size of the particles.

To synthesize the particles, the team used a microwave-assisted hydrothermal method that operates at relatively low temperatures. They prepared solutions containing lead nitrate as the lead source and potassium fluoride as the fluoride source. For the titanium source, they tested three different water-soluble titanium complexes based on citric acid, tartaric acid, and lactic acid. They also prepared a conventional PTOF sample using titanium chloride (TiCl4) for comparison. The mixtures were heated in a microwave at 473 K, and the resulting precipitates were collected and dried.
The PTOF particles synthesized using suitable water-soluble titanium complexes were smaller (less than 100 nm) and featured highly porous structures with surface areas of ~40 m2g-1. In comparison, particles obtained using TiCl4 were larger (0.5–1 μm) and exhibited a surface area of just 2.5 m2g-1.
This nanostructuring significantly improved photocatalytic activity. For hydrogen generation, citric acid-derived PTOF showed a sixty-fold increase in reaction rate compared to PTOF prepared with TiCl4, achieving an efficient quantum yield of approximately 15% at 420 nm. For CO2 reduction, tartaric acid-derived PTOF performed best, producing formic acid with a promising quantum yield of approximately 10% in the presence of a molecular ruthenium photocatalyst—both values representing record highs for oxyhalide photocatalysts.
Interestingly, the researchers observed that the smaller particles had lower charge carrier mobility than their larger counterparts. However, because the carriers in these nanosized structures had a much shorter distance to travel to reach the surface, they were less likely to recombine and more likely to take part in useful chemical reactions.
By using a low-temperature, eco-friendly method and carefully controlling the size and structure of the particles, the researchers have developed a practical approach for scalable and efficient solar fuel production.
"This study underscores the importance of controlling the morphology of oxyhalides to unlock their full potential as photocatalysts for artificial photosynthesis. These findings are expected to significantly contribute to the development of innovative materials that help address global energy challenges," concludes Maeda.
More information: Hiroto Ueki et al, Mesoporous Oxyhalide Aggregates Exhibiting Improved Photocatalytic Activity for Visible-Light H2 Evolution and CO2 Reduction, ACS Catalysis (2025).
Journal information: ACS Catalysis
Provided by Institute of Science Tokyo