Optical tweezer sectioning microscopy enables 3D imaging of floating live cells

Sadie Harley
scientific editor

Robert Egan
associate editor

Three-dimensional (3D) imaging is essential for investigating cellular structure and dynamics. Traditional optical methods rely on adhesive or mechanical forces to hold and scan cells, which limit their applicability to suspended cells and may induce stress responses. Developing a non-contact, all-optical 3D imaging technique for live suspended cells remains a major challenge in advancing in situ biological research.
In a study published in , Prof. Yao Baoli from the Xi'an Institute of Optics and Precision Mechanics (XIOPM) of the Chinese Academy of Sciences and Prof. Olivier J. F. Martin, from the Swiss Federal Institute of Technology, Lausanne, developed the optical tweezer sectioning microscopy (OTSM), enabling all-optical 3D imaging of suspended live cells, which offers a powerful new tool for live-cell imaging, dynamic biological studies and multicellular assembly.
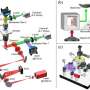
Researchers developed OTSM by integrating holographic optical tweezers (HOTs) with structured illumination microscopy (SIM).
An array of petal-shaped optical traps accurately captured multiple suspended live yeast cells. Axial scanning achieved full volume imaging, capturing three phase-shifted images at each depth. SIM reconstruction produced high-resolution slices, achieving contact-free, high-fidelity 3D reconstruction without sample fixation.
The OTSM enabled precise geometric trapping of 12 suspended live yeast cells into hexagonal, pentagonal and ring shapes followed by clear 3D imaging, demonstrating full optical integration from manipulation to reconstruction.
HOT effectively trapped cells within structured illumination stripe periods, significantly reducing motion blur and ensuring stable axial scanning.
Reconstructed images revealed distinct cellular features with dark shells enclosing bright cores. The average shell diameter was 4.16 μm laterally and 6.21 μm axially while the core measured 2.72 μm laterally and 4.34 μm axially, with ellipticities being 0.67 and 0.63 respectively.
The OTSM technology overcomes the limitations of conventional bioimaging techniques that rely on static samples and mechanical scanning.
"It promotes the integration of structured illumination microscopy and optical manipulation, and the cross-disciplinary fusion of optical tweezers with other imaging techniques to meet the demands for isotropic resolution, large field of view, and super-resolution imaging," said Prof. Yao Baoli.
More information: Xing Li et al, Optical tweeze-sectioning microscopy for 3D imaging and manipulation of suspended cells, Science Advances (2025).
Journal information: Science Advances
Provided by Chinese Academy of Sciences