Alternating pulses enhance copper's role in converting COâ‚‚ to valuable fuels

Gaby Clark
scientific editor

Robert Egan
associate editor

Scientists from the Interface Science Department at the Fritz Haber Institute have studied how applying pulsed electric potential treatments to copper single crystal surfaces as model catalysts can improve their ability to convert carbon dioxide (CO2) into fuels like ethylene and ethanol. The key to achieving selectivity tunability relies on the control of the pulsed-induced structural and chemical catalyst transformations. This research offers insights which could help to reduce CO2 emissions and produce renewable energy sources.
The study is in the journal Nature Catalysis.
The rapid industrialization and deforestation worldwide have led to a significant increase in carbon dioxide (CO2) emissions, a major contributor to global climate change. Addressing this issue requires innovative solutions to reduce emissions as well as to convert the still irremediably produced CO2 into useful products. Copper has emerged as a promising catalyst for this conversion, particularly in forming valuable chemical compounds like ethylene and ethanol.
The team led by Dr. Thomas Schmidt and Prof. Beatriz Roldán Cuenya has applied a recently developed method using pulsed potentials in electrochemical treatments combined with in-depth spectro-microscopy characterization methods (LEEM/XPEEM) to understand and ultimately tune the electro-catalytic properties of well-defined copper surfaces.
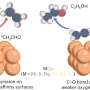
By applying alternating anodic (oxidizing) and cathodic (reducing) pulses, they observed that copper surfaces undergo changes in their structure (formation of specific crystalline facets) and oxidation state (generation and stabilization of Cu(I) species) that result in a more efficient conversion of CO2 into hydrocarbons and alcohols.

Understanding the science
The study employed advanced spectro-microscopy techniques to observe these changes at a microscopic level. The researchers found that the pulsed treatments create two kinds of unique surface structures on copper. During the anodic pulse, inverted pyramid like structures with specific side facets are formed by site-selective dissolution of copper into the electrolyte. Furthermore, at this anodic pulse (+0.6 V), the copper surface is oxidized, resulting in an about 1 nm thick film of Cu(I).
Interestingly, at the following cathodic pulse (-1 V) only the topmost part of this film is reduced to metallic Cu, giving rise to a sandwich-like structure of a ~0.5 nm thick metallic copper film on a ~0.5 nm thick Cu(I) subsurface layer on the metallic copper bulk crystal. Both structures, the facets and the subsurface oxide are important for the enhanced production of ethylene and ethanol.
In particular, the coexistence of metallic and Cu2O species appears to enhance ethanol production, while stepped mainly metallic surfaces lead to enhanced ethylene yields. This insight provides valuable feedback for theoretical models and helps refine the understanding of copper's catalytic behavior.
This research offers a promising avenue for developing sustainable energy solutions. By improving the efficiency of CO2 conversion, the findings could lead to more effective ways of reutilizing "climate-killer" greenhouse gases such as carbon dioxide for the production of renewable fuels. The innovative use of pulsed electric potential treatments on copper surfaces represents a step forward in the quest for cleaner energy technologies.
More information: Liviu C. Tănase et al, Morphological and chemical state effects in pulsed CO2 electroreduction on Cu(100) unveiled by correlated spectro-microscopy, Nature Catalysis (2025).
Journal information: Nature Catalysis
Provided by Max Planck Society