Atomic-level simulations reveal new class of protein misfolding in high definition

Sadie Harley
scientific editor

Robert Egan
associate editor

New computer simulations that model every atom of a protein as it folds into its final three-dimensional form support the existence of a recently identified type of protein misfolding.
Proteins must fold into precise three-dimensional shapes—called their native state—to carry out their biological functions. When proteins misfold, they can lose function and, in some cases, contribute to disease.
The newly spotted misfolding results in a change to a protein's structure—either a loop that traps another section of the protein forms when it shouldn't or doesn't when it should—that disrupts its function and can persist in cells by evading the cell's quality control system.
The simulated misfolds also align closely with structural changes inferred from experiments that track protein folding using mass spectrometry, according to the team led by researchers at Penn State.
"Protein misfolding can cause disease, including Alzheimer's and Parkinson's, and is thought to be one of the many factors that influence aging," said Ed O'Brien, professor of chemistry in the Eberly College of Science, a co-hire of the Institute for Computational and Data Sciences at Penn State and the leader of the research team.
"This research represents another step forward in our attempt to document and understand the mechanisms of protein misfolding. Our aim is to translate these fundamental discoveries into therapeutic targets that could help mitigate the impacts of these disorders and even aging."
A describing the research appeared in the journal Science Advances.
Proteins are composed of long strings of units called amino acids. A protein's function relies on the sequence of those amino acids along the string, which determines how the string will fold into a three-dimensional structure.
Sections of the protein can fold into helices, loops, sheets and various other structures, which allow them to interact with other molecules and perform their functions. Any mistake during this folding process can disrupt these functions.
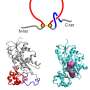
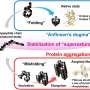
The new class of misfolding, identified by the O'Brien Lab, involves a change in entanglement status in the protein's structure. Entanglement refers to sections of the string of amino acids looping around each other like a lasso or a knot. Sometimes an entanglement can form when it shouldn't be there and sometimes an entanglement that is part of the protein's native structure doesn't form when it should.
"In our previous study, we used a coarser-grained simulation that only modeled the protein at the amino acid level, not the atomic level," said Quyen Vu, first author of the paper and a postdoctoral researcher in chemistry at Penn State who started the research as a graduate student at the Polish Academy of Sciences.
"But there was concern in the community that such a model might not be realistic enough, as the chemical properties and bonding of the atoms that make up amino acids influence the folding process. So, we wanted to make sure we still saw this class of entanglement misfolding with higher-resolution simulations."
The team first used all-atom models of two small proteins and simulated their folding. They found that both small proteins could form the misfolds just like in their coarser-grained simulations. However, unlike in their previous simulations, which modeled normal-sized proteins, the misfolds in these small proteins lasted only a short time.
"We think that the misfolds in our previous simulations persisted for two main reasons," Vu said.
"First, to fix the misfold required backtracking and unfolding several steps to correct entanglement status, and second, the misfold can be buried deep inside the protein's structure and essentially invisible to the cell's quality control system. With the small proteins, there were fewer steps and less to hide behind, so the mistakes could be quickly fixed. So, we simulated a normal-sized protein at the atomic scale and saw misfolding that persisted."
The team also tracked folding of the proteins used in their simulations experimentally. While they couldn't directly observe the misfolds in the experiments, structural changes inferred using mass spectrometry occurred in the locations that misfolded in their simulations.
"Most misfolded proteins are quickly fixed or degraded in cells," O'Brien said.
"But this type of entanglement presents two major problems. They are difficult to fix as they can be very stable, and they can fly under the radar of the cell's quality control systems. Coarse-grain simulations suggest that this type of misfolding is common.
"Learning more about the mechanism can help us understand its role in aging and disease and hopefully point to new therapeutic targets for drug development."
More information: Quyen Vu et al, Non-native entanglement protein misfolding observed in all-atom simulations and supported by experimental structural ensembles, Science Advances (2025). .
Journal information: Science Advances
Provided by Pennsylvania State University