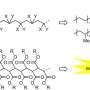
Vinyl polymerization and C1 polymerization. Credit: Macromolecules (2025). DOI: 10.1021/acs.macromol.5c00704
The most common method for synthesizing polymers with a carbon–carbon main chain backbone is vinyl polymerization, by which many industrially important polymers, including common plastics such as polyethylene and polypropylene, are synthesized. This method uses the reaction of a C=C double bond of vinyl compounds used as monomers, and thus the main chain backbone is generated from two-carbon units.
Conversely, C1 polymerization is a complementary method for preparing carbon–carbon main chain polymers, where the carbon-based backbone is constructed from one-carbon units by utilizing the unique reactivity of monomers such as diazoacetate and sulfoxonium methylide.
Therefore, functional groups derived from the monomer can be introduced at every main chain carbon atom, and characteristic properties and functions are expected to emerge due to higher accumulation of the functional groups compared to the corresponding vinyl polymers bearing the same functional groups on every other carbon atom.
A team from Ehime University previously succeeded in developing "C1 cyclopolymers" by conducting cyclopolymerization (additional polymerization that proceeds while forming cyclic structures in the main chain) of bifunctional diazoacetates.
These C1 cyclopolymers have a unique structure, where all the main chain carbons are incorporated into a cyclic framework. Their physical properties are of interest because they have a structure in which cyclic repeating units are more densely accumulated than cyclopolymers obtained from the cyclopolymerization of corresponding divinyl compounds (C2 cyclopolymers).
However, due to the synthetic difficulty of bifunctional diazoacetates, there were limitations on the synthesizable polymer structures.
In a , now published in Macromolecules, the Ehime team attempted to develop a new synthetic method for bifunctional diazoacetates that overcomes these limitations and to perform C1 cyclopolymerization of the resulting monomers. As a result, they succeeded in developing new monomer syntheses using pentaerythritol, one of the polyhydric alcohols, as a starting material.
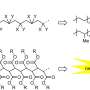
Synthesis and C1 cyclopolymerization of bifunctional diazocarbonyl compounds. Credit: Macromolecules (2025). DOI: 10.1021/acs.macromol.5c00704
By using bifunctional diazoacetates obtained with these methods, the team succeeded in synthesizing novel C1 cyclopolymers with diverse ring sizes (9- to 19-membered rings) and functional groups (such as urethane linkage capable of forming hydrogen bonds within the cyclic framework).
Furthermore, investigation of the thermal properties of a series of the obtained C1 cyclopolymers revealed that they possessed significantly higher glass transition temperatures compared to the corresponding C1 polymers without cyclic structures.
These results provide new insights into the molecular design of carbon–carbon main chain polymers and are expected to lead to new polymer materials with improved properties.
More information: Hiroaki Shimomoto et al, C1 Cyclopolymerization of Bis(diazocarbonyl) Compounds Derived from Pentaerythritol, Macromolecules (2025).
Provided by Ehime University