Researchers achieve light-induced heterolytic hydrogen dissociation at ambient temperature

Sadie Harley
scientific editor

Robert Egan
associate editor

In a study published in Science, a research team led by Prof. Wang Feng from the Dalian Institute of Chemical Â鶹ÒùÔºics (DICP) of the Chinese Academy of Sciences, along with Prof. Paolo Fornasiero from the University of Trieste in Italy, has developed a photochemical strategy for heterolytic hydrogen (H2) dissociation at ambient temperature, a long-standing challenge in H2 activation chemistry.
Hydrogenation is one of the most fundamental reactions in the chemical industry. It is estimated that 25% of all chemical processes include at least one hydrogenation step. An essential procedure of hydrogenation is H2 dissociation. It occurs through two pathways: homolytic and heterolytic dissociation.
Heterolytic H2 dissociation stands out for producing many fine chemicals since it generates reactive, polar H2 species that can selectively reduce polar functional groups. However, it typically operates at high temperatures and pressures, leading to high energy consumption and safety concerns.
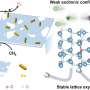
In this study, the researchers developed a light-induced strategy to realize heterolytic H2 dissociation. Using gold-loaded titanium dioxide (Au/TiO2) as a model photocatalyst, the researchers showed that upon ultraviolet (UV) irradiation, electrons migrated from TiO2 to gold (Au) nanoparticles, and holes were captured at the interfacial defects constituted by Au–O–Ti scaffolds.
This spatial proximity of electrons to Au and holes at interfacial defects formed electron-hole pairs that drove the heterolytic H2 dissociation. The activity of the heterolytic H2 dissociation scaled almost linearly with light intensity, confirming the photocatalytic nature of H2 dissociation process.
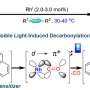
Furthermore, the researchers demonstrated the advantages of this strategy. They reduced the inert carbon dioxide (CO2), and showed that the dissociated H2 species almost fully reduced CO2 into ethane at ambient temperature. Furthermore, cascading with a subsequent photocatalytic ethane dehydrogenation generated ethylene with >99% yield over 1,500 h of UV irradiation.
The light-induced H2 dissociation is universal and can be extended to visible-light-responsive photocatalysts, such as Au/N-doped TiO2, Au/CeO2, and Au/BiVO4. A demonstration utilizing solar energy to convert CO2 achieved an ethane selectivity of up to 90%.
"This work offers a promising route to produce high-value chemicals like ethane and ethylene from H2 and CO2 under ambient conditions, which could lower the energy cost and reduce carbon emissions," said Prof. Wang.
"In the future, we hope the strategy develops into a scalable, sunlight-driven or photothermal coupled technique to upgrade modern coal-based chemical industries."
More information: Photochemical H2 dissociation for nearly quantitative CO2 reduction to ethylene, Science (2025).
Journal information: Science
Provided by Dalian Institute of Chemical Â鶹ÒùÔºics, Chinese Academy Sciences