Key transition point in catalyst kinetics could boost green hydrogen production

Gaby Clark
scientific editor

Robert Egan
associate editor

Researchers from the Fritz Haber Institute of the Max Planck Society have unveiled new insights into the activity of catalysts used in green hydrogen production.
Their study, in Nature Chemistry, explores how the catalyst kinetics are related to an intricate interplay between interfacial solvent and chemical changes on the catalyst surface, potentially paving the way for more efficient energy conversion technologies.
The Department of Interface Science at the Fritz Haber Institute has made significant strides in understanding how catalysts function in aqueous environments. This research is crucial for advancing technologies like green hydrogen production, which relies on efficient catalysts to split water molecules.
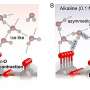
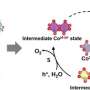
The study, led by Dr. Martinez-Hincapié and Dr. Oener within Prof. Roldán Cuenya's FHI department utilizes temperature-dependent electrochemistry and operando spectroscopy to investigate the oxygen evolution reaction (OER). This reaction is a bottleneck in water electrolysis, where sluggish OER kinetics can hinder hydrogen production. The researchers discovered a key transition point in bias-dependent kinetics where the catalyst's activity shifts from being limited by the buildup of excess charge to becoming highly active.
A critical finding of the study is the role of interfacial solvation—a process by which ions are losing or gaining solvent molecules. This initial step appears to be crucial for the catalyst's intrinsic activity.
Dr. Oener explains, "We should really think about the catalyst-electrolyte interphase as a whole, not in separate terms. We cannot understand the stabilization of excess charge on the solid side without the response of the solvent and we cannot understand interfacial ion solvation without carefully considering what happens on the solid side.
"This is particularly critical since the solid interface also experiences drastic structural and chemical changes in the course of the reaction. It is one interphase that gives rise to the observed activity."

In more technical terms, the study reveals that the catalyst's kinetics are governed by an intricate interplay between chemical and structural adaptation of the oxide surface and the response of interfacial water molecules. Using operando X-ray spectroscopy, the team observed structural and chemical adaptations of the oxide catalysts right at an important transition potential in the kinetics.
Importantly, this transition potential is not dependent on the catalyst loading (the amount of material used), nor its surface area. This indicates that the catalyst's activity is intrinsically linked to its ability to build up excess charge to interact with solvated ions from the liquid electrolyte.
Prof. Dr. Beatriz Roldán Cuenya highlights the importance of combining different operando spectro-microscopy techniques that inform the catalyst surface, the solvent and the fundamental kinetics. This is needed to gain deeper insights into the catalyst behavior.
The research not only advances our understanding of the catalyst activity, but also holds promise for improving energy conversion technologies. The team is committed to further exploring these findings, with the potential to significantly impact the fields of energy and chemical conversion technology.
More information: Ricardo MartÃnez-Hincapié et al, Interfacial solvation pre-organizes the transition state of the oxygen evolution reaction, Nature Chemistry (2025).
Journal information: Nature Chemistry
Provided by Max Planck Society