How cells remodel their membranes—without any energy supply

Gaby Clark
scientific editor

Robert Egan
associate editor

For life to function, cell membranes must remain intact. When these fragile barriers are damaged—for instance by heat or viral attack—specialized proteins come to the rescue. Researchers at Forschungszentrum Jülich, Heinrich Heine University Düsseldorf and the University of Mainz have now, for the first time, uncovered the mechanism of action of one such protective protein, PspA, which belongs to the ESCRT-III superfamily.
Their study, in Proceedings of the National Academy of Sciences, reveals how the protein actively reshapes cell membranes—without requiring any external energy. The findings shed light on a fundamental biological repair process.
PspA is part of an ancient protein family—the ESCRT-III superfamily—that sculpts membranes across all domains of life. In bacteria, PspA helps safeguard the inner membrane under stress conditions. Earlier work by the team had already shown PspA's structural relationship to its eukaryotic counterparts, including those in humans, and described its role in membrane repair. The new study now adds a crucial missing piece.
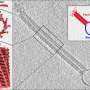
By combining cryo-electron microscopy with molecular dynamics simulations, the scientists were able to show how PspA assembles into tube-like protein complexes that bend, constrict, and eventually transform parts of the membrane into small vesicles.
"At the core of the process is a tiny helix structure that embeds itself in the membrane surface and deforms it," explains Esther Hudina of the Ernst Ruska-Center for Microscopy and Spectroscopy with Electrons (ER-C-3) at Forschungszentrum Jülich, one of the study's first authors. Cryo-electron microscopy at the Ernst Ruska-Center provided static snapshots of the molecules in solution.

"In this way, PspA can enclose, reshape, and pinch off damaged membrane sections—most likely as part of the repair process," adds bioinformatician Dr. Stephan Schott-Verdugo from the Institute of Bio- and Geosciences (IBG-4).
Molecular dynamics simulations on Jülich's JUWELS supercomputer, carried out at IBG-4 under the direction of Prof. Holger Gohlke, who is also a professor at Heinrich Heine University Düsseldorf, made the movements of the molecules visible on the computer. They also provided a model of how the energy required for membrane curvature can be generated by the progressive helix-membrane interactions.
"We were struck by the fact that this entire mechanism works without any external energy input," says Prof. Carsten Sachse, senior author and head of the Ernst Ruska-Center for Microscopy and Spectroscopy with Electrons in Structural Biology (ER-C-3). "The driving force comes solely from the protein's binding to the membrane—a remarkable biological trick."
Beyond advancing our basic understanding of cell biology, the study also points to potential biotechnological applications—for example in the targeted delivery of therapeutics using artificial vesicles.
More information: Esther Hudina et al, The bacterial ESCRT-III PspA rods thin lipid tubules and increase membrane curvature through helix α0 interactions, Proceedings of the National Academy of Sciences (2025).
Journal information: Proceedings of the National Academy of Sciences
Provided by Jülich Research Centre