Chemists detail new photobiocatalytic approach to carbon-nitrogen bond formation

Gaby Clark
scientific editor

Robert Egan
associate editor

All life on Earth depends on enzymes—natural proteins—that act as catalysts to hasten chemical reactions and keep biological processes functioning.
Carbon-nitrogen bond formation is among the catalytic-dependent processes needed to enable the cycling of nitrogen through ecosystems and the constant formation of complex structures that sustain plant and animal life.
Often, though, Mother Nature needs a boost and human-made synthetic catalysts supplement the insatiable global demand for food, energy and pharmaceutical production. A key challenge to this endeavor is developing catalysts capable of making chemical bonds needed for these processes in the most energy-efficient and environmentally friendly methods possible.
"Copper-based catalysts, created using readily available components, have been studied for this purpose over the past decade, but successfully achieving such copper-based catalysis in enzymes remains elusive," says Utah State University chemist Yi Rao.
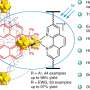
"Our research group, together with our collaborators, introduces a photobiocatalytic approach that bypasses the need for fossil fuels, using a copper-substituted nonheme enzyme."
Rao and Jesse Brown of Utah State, along with colleagues Xuzhong Shen, lead author; Xiongyi Huang, lead corresponding author; James Zhang, Xinyuan Ji and Jinyan Rui from Johns Hopkins University, as well as collaborators from China's Zhejiang University of Technology and Spain's University of Girona, detail this approach in the journal .
Shen says an exciting aspect of the study is replacing the iron center, which is native to this enzyme, with copper.
"By performing this single-atom-level surgery, we can preserve the versatility of the protein scaffold, while completely transforming its chemical reactivity profile," he says.
Rui says this step is particularly important because nonheme iron enzymes are far more diverse and engineerable than natural copper-based enzymes.
"Our approach could potentially convert thousands of nonheme iron enzymes into copper-based biocatalysts," she says.
Rao, associate professor in USU's Department of Chemistry and Biochemistry, says the team is still trying to understand 'the why' of chemical reactions and how these reactions play a role in photocatalysis.
"Our aim is to mimic nature and foster the development of novel, nature-based pharmaceuticals," says Rao.
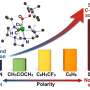
Rao says more than 80% of commonly used pharmaceuticals, including antibiotics, antivirals, statins and antacids, require the formation of at least one C-N bond during production.
"By developing a more sustainable and energy-efficient catalyst for C-N bond formation, we are promoting more energy-efficient and cost-effective production of life-saving pharmaceuticals," he says, in many biologically active compounds.
More information: Xuzhong Shen et al, Enantioconvergent benzylic C(sp 3 )‒N coupling with a copper-substituted nonheme enzyme, Science (2025).
Journal information: Science
Provided by Utah State University