First comprehensive transport model of a plasma membrane calcium pump could inform new drug development

Lisa Lock
scientific editor

Robert Egan
associate editor

We've all heard the advice: "Drink your milk and you will have strong bones and healthy teeth." It's supposed to help us meet our bodies' high calcium requirements. However, our cells keep calcium levels as low as possible at all times. They achieve this by literally pumping calcium ions out of their interior using high-speed pumps in their membrane.
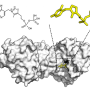
Now, a team of researchers led by Stefan Raunser, director at the Max Planck Institute of Molecular �鶹��Ժiology in Dortmund, and Bernd Fakler, Director at the Faculty of Medicine at the University of Freiburg, has succeeded in establishing the first comprehensive transport model of the plasma membrane Ca²⁺-ATPase (PMCA) by resolving its 3D structure in various states of activity and tracking PMCA-mediated Ca2+ pumping in intact cells.
The researchers were thus able to show that its high speed is primarily due to interactions with the plasma membrane lipid PIP2. This mechanism could be a promising starting point for developing new drugs that manipulate calcium concentrations in cells. Their study is in Nature.
Our body contains approximately 1 kg of calcium, most of it stored in bones and teeth. A small portion of calcium plays a crucial role for controlling a broad variety of cellular processes such as muscle contraction, signal transduction in neurons, mitosis, gene expression and cell signaling. Calcium levels must be precisely controlled, as imbalances can lead to symptoms of disease.
"The differences in concentration between the extracellular space and intracellular milieu are immense: calcium levels inside the cell can be up to 50,000 times lower than outside the cell," says Fakler. This steep concentration gradient is fundamental to the speed and effectiveness of calcium-mediated signaling in the cell. The gradient literally pushes calcium ions into the cell upon opening of calcium channels, and even the smallest additional quantities make a noticeable difference.
Pushing calcium back out of the cell is an exhausting endeavor
"Cells have to invest a lot of energy to maintain this concentration gradient, especially when it comes to getting rid of calcium," says Raunser. "It's like being on a rush-hour train in a city like Tokyo. When the doors of a crowded train open at an empty station, people literally burst out. Conversely, getting into a crowded train requires a lot of strength."
In Japan, commuters are packed into rush-hour trains by special staff called "pushers." Such pushers also exist in the cell: Calcium pumps actively push calcium ions out against the steep concentration gradient using the cellular energy source ATP. Remarkably, they do this at a speed of 5,000 calcium ions per second. The way in which this speed and efficiency is maintained has so far remained elusive.
Keeping calcium tight and releasing it quickly
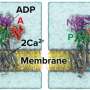
By combining their expertise, researchers from Dortmund and Freiburg have now established the first comprehensive model of how calcium is transported out of the cell by the plasma membrane Ca²⁺-ATPase. The Dortmund team used electron cryo-microscopy to resolve the pump's structure in various states occurring during calcium transport, while the Freiburg team recorded the pump's activity in living cells.
The researchers revealed that the calcium pump's high speed is enabled by several features. First, calcium binds very tightly to the pump, facilitating initiation. Second, the calcium pump exhibits only minor structural changes in its cytoplasmic domains and minimal interaction areas between these domains during the calcium ion transport cycle, unlike other slower pumps. These smooth and accelerate the transitions between states, ultimately increasing the speed of the pump.
The most important factor, however, is the pump's interaction with PIP2, a lipid found in the cell's plasma membrane. The researchers demonstrated that PIP2 stabilizes calcium binding but also facilitates its rapid release, making it the pump's primary acceleration factor.
A new starting point for therapeutic exploitation
"Although calcium plays such an important role in health and diseases such as Alzheimer's, Parkinson's, heart failure, diabetes and cancer, there are currently very few strategies or drugs available to influence calcium levels in cells," says Raunser.
"Targeting calcium homeostasis and signaling for cancer therapy has become an emerging research field, but few agents have progressed to clinical trials, and all of these target calcium channels or calcium pumps other than PMCA.
"Interestingly, we have found that the PIP2 binding site represents a druggable target for manipulating PMCA's activity. This opens up new possibilities for developing innovative drugs that can either increase the concentration of calcium in cells or induce cell death in targeted cancer therapy."
More information: Deivanayagabarathy Vinayagam et al, Molecular mechanism of ultrafast transport by plasma membrane Ca2+-ATPases, Nature (2025).
Journal information: Nature
Provided by Max Planck Society