Glycerol reveals previously overlooked connections between intracellular metabolism and vesicle transport

Lisa Lock
scientific editor

Robert Egan
associate editor

Prof. Fang-Jen S. Lee's team at National Taiwan University has discovered that an intermediate cellular metabolism molecule, glycerol, regulates the localization and function of the Golgi protein Imh1, thus revealing a previously unappreciated connection between cellular metabolism and vesicular transport.
The organelles within cells, composed of biological membranes, rely on vesicle transport to achieve nutrient exchange, signal transmission, recycling of cellular materials, and waste processing, akin to the importance of the circulatory system in the human body. To date, nearly a hundred genetic, autoimmune diseases, and cancers have been closely linked to disruptions in vesicle transport mechanisms.
Recently, the research team led by Prof. Lee discovered that glycerol within cells can regulate the localization and function of the Golgi structural protein Imh1, further revealing the molecular regulatory mechanisms between cellular metabolism and vesicle transport pathways. This research has been in Nature Structural & Molecular Biology.
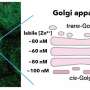
The Golgi apparatus is a crucial organelle in eukaryotic cells responsible for protein modification and vesicle transport, known as the "transport center" within cells. Its normal functioning is vital for the accurate delivery of proteins to specific organelles. If its transport function is abnormal, it may lead to neurological degradation, developmental disorders, autoimmune diseases, and cancer.
Professor Lee's team has long been dedicated to exploring the molecular regulatory mechanisms of Golgi vesicle transport. Previous studies have indicated that the Golgi protein Imh1 is involved in maintaining the structural integrity and transport function of the Golgi apparatus, although the detailed regulatory mechanisms were unclear.
The researchers first discovered that when cells encounter hypotonic shock and intracellular glycerol levels decrease, the localization of Imh1 and the integrity of the Golgi apparatus are disrupted. Furthermore, the study confirmed that metabolic abnormalities and reduced glycerol levels prevent Imh1 from correctly localizing to the Golgi apparatus, leading to transport dysfunction.
To delve into the molecular basis of this phenomenon, Professor Lee's research team collaborated with Professor Yu Chia-Jung's proteomics team from Chang Gung University. They employed advanced isotope-labeled cross-linkers with cross-linking mass spectrometry (XL-MS) to conduct quantitative analysis of lysine cross-linking site alterations, proving glycerol's regulatory effect on the Imh1 protein configuration.
This regulatory mechanism is observed not only in yeast but also extends to mammalian cells, indicating its evolutionary conservation and importance.
Since glycerol is an important intermediary metabolite within cellular metabolism, this study reveals previously overlooked connections between intracellular metabolism and vesicle transport. It further emphasizes the critical role of metabolic pathways in maintaining intracellular transport functionality.
"Intracellular glycerol levels might be associated with various vesicle transport diseases, making the exploration of its mechanisms potentially valuable for discovering new therapeutic strategies with clinical applications," said Prof. Lee.
More information: Wan-Yun Chiu et al, Glycerol mediates crosstalk between metabolism and trafficking through the golgin Imh1, Nature Structural & Molecular Biology (2025).
Journal information: Nature Structural & Molecular Biology
Provided by National Taiwan University