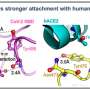
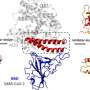
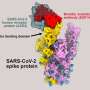
The illustration depicts an allosteric inhibitor bound to ACE2, blocking the interaction between the SARS-CoV-2 Spike protein and ACE2. Simultaneously, the inhibitor enhances the binding of ACE2 to its natural substrate, Angiotensin II, preserving its physiological function. Credit: Saroj Kumar Panda
Early in the pandemic, most research, including our own, focused on designing drugs that could block the virus's spike protein. This was a logical first step, but as we've seen, the virus is a moving target. It was rapidly evolving, and new variants acquired resistance due to changes in the surface spike glycoprotein (S protein).
This highlighted a critical challenge: would our treatments still work as the virus continued to change? Instead of constantly chasing new variants, we began to ask, what if we focused on how the human body responds to the virus, rather than only targeting the virus itself?
This simple but powerful idea became the focus of our research, which we're proud to have recently in the Â鶹ÒùÔºical Chemistry Chemical Â鶹ÒùÔºics journal.
Shifting focus: Targeting the host, not the virus
Instead of pursuing the virus directly, we decided to explore a new idea: targeting the human protein that mediates the virus's entry into cells in our body. This led us to angiotensin converting enzyme-2 (ACE2), the critical "gateway" protein the virus hijacks to begin its invasion. ACE2 is present on the surface of many human cells, especially in the lungs, and plays a crucial role in regulating blood pressure and heart health. Unfortunately, SARS-CoV-2 hijacks this protein as its entry point into cells.
This poses a significant challenge: blocking ACE2 entirely isn't a good option, as it's far too important for normal body functions. So our goal was: can we make it harder for the virus to use ACE2 without disturbing its vital role in our bodies?
Finding a hidden switch
To answer this, we used a combination of powerful Computational Chemistry techniques. Our research adventure strayed from conventional approaches by not attempting to block the viral-binding domain of ACE2. Instead, we used a computational allostery approach to discover the protein's hidden "allosteric" site. This allosteric site acts as a kind of molecular switch that, when triggered, can modulate the way the whole protein behaves.
Using molecular dynamics (MD) simulations, we could visualize and study ACE2 and the virus's interaction at an atomic level. Our simulations showed that a small molecule modulator could bind to this newly discovered allosteric pocket, which is located away from the primary virus-interacting site. The binding free energies calculated using the MM/PBSA method confirmed that our lead compounds bind favorably to this allosteric pocket, demonstrating the thermodynamic feasibility of our approach.
(A) The binding pose of SB27012 in the hACE2–spike RBD complex and a magnified representation of the designated allosteric pocket, (B) binding poses of three SB27012 molecules at 0 and 1000 ns as derived from an unbiased MD simulation, and (C) their RDF and distance analyses with Respect to the designated residues. Credit: Â鶹ÒùÔºical Chemistry Chemical Â鶹ÒùÔºics (2025). DOI: 10.1039/D5CP01740H
The double-duty advantage: Hindering the virus while helping the host
When a suitable small molecule binds to this allosteric pocket of hACE2, it causes a conformational change in ACE2. This change primarily affects the global allostery, which is critical for the protein's interaction with the viral spike glycoprotein. This conformational shift weakens the binding between ACE2 and the viral spike protein, making it tougher for the virus to latch on and infect a cell.
The real innovation, however, is that this conformational change does not inhibit ACE2's normal function; in fact, our simulations and calculations confirmed that it enhances it. The allosteric modulation increases ACE2's catalytic activity with its natural substrate, Angiotensin II (AngII), a key part of regulating blood pressure. So, instead of blocking ACE2, we gently "nudge" it in a way that both helps us and hinders the virus.
This mechanism is analogous to tuning a lock: the original key (Angiotensin II) fits even better, but a copy (the virus) also no longer works. Our work demonstrated that this allosteric modulation strategy could be an effective way to inhibit viral entry.
Looking ahead: A more resilient defense
The advantage of this host-targeted approach is significant. Most antiviral strategies aim to block the virus itself, but viruses like SARS-CoV-2 mutate rapidly; a drug that works today may fail tomorrow. Targeting the human proteins viruses depend on makes it much harder for viruses to escape treatment.
This makes our approach potentially more durable against future variants of concern. By understanding and strategically manipulating our own cellular machinery, we can build a more resilient defense against viral threats.
A team effort and a personal journey
I am especially proud that this research was carried out at IISER Berhampur, a young and growing research Institute in India. Our lab, the Â鶹ÒùÔºical Biomolecular Research Lab, employs advanced computational tools to explore the therapeutic design against different pathogenic targets.
This work highlights the power of thinking differently and underscores the value of fundamental science in addressing real-world challenges. It was a true team effort involving me, Pratyush Pani (Ph.D. scholar), and our group leader, Dr. Malay Kumar Rana. Together, we combined our expertise and curiosity to push the boundaries of what is possible through Computational Biology.
Science doesn't always need to fight hard; it just needs to be smart.
This story is part of , where researchers can report findings from their published research articles. for information about Science X Dialog and how to participate.
More information: Pratyush Pani et al, Modulating functional allostery of the host-cell receptor protein hACE2 to inhibit viral entry of SARS-CoV-2, Â鶹ÒùÔºical Chemistry Chemical Â鶹ÒùÔºics (2025).
Journal information: Â鶹ÒùÔºical Chemistry Chemical Â鶹ÒùÔºics
Dr. Saroj Kumar Panda is a Postdoctoral Research Associate in the Department of Chemistry and Biochemistry at the University of Texas at Arlington, USA. His research centers on therapeutic design targeting various pathogenic targets and exploring enzymatic reaction mechanisms using advanced computational techniques. Dr. Panda earned his Ph.D. from the Department of Chemical Sciences, Indian Institute of Science Education and Research (IISER) Berhampur under the mentorship of Dr. Malay Kumar Rana.