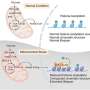
The model of LIN-40 sensing mitochondrial stress via phosphorylation of T654. Credit: IGDB
A new study by Dr. Tian Ye's group from the Institute of Genetics and Developmental Biology (IGDB) of the Chinese Academy of Sciences (CAS) has revealed that mitochondrial stress coordinates chromatin remodeling and longevity through the phosphoregulation of Nucleosome Remodeling and Deacetylase (NuRD) complex component LIN-40 in Caenorhabditis elegans.
This study for the first time clarifies the molecular mechanism by which the NuRD complex senses mitochondrial signals, providing a new perspective for understanding mitochondria-to-nucleus signaling and aging regulation. The results were in Science China Life Sciences on August 13.
Mitochondria are widely known as the energy centers of cells, but they can also serve as signaling hubs that influence aging, immunity, cell proliferation, and apoptosis. Intriguingly, previous studies in multiple model organisms, including Caenorhabditis elegans, Drosophila melanogaster, and Mus musculus, have consistently shown that mild mitochondrial stress can promote organismal longevity. In C. elegans, such stress activates transcriptional responses in the nucleus, promoting the repair of mitochondrial damage, reprogram metabolism, and stress adaptation.
A previous study by Tian's lab demonstrated that the NuRD complex, an epigenetic regulator of chromatin structure, plays a key role in mediating mitochondrial stress-induced longevity. However, how the NuRD complex senses mitochondrial signals remains unclear.
To solve this puzzle, the researchers purified the NuRD complex under mitochondrial stress conditions and conducted a proteomic analysis. They identified stress-induced dephosphorylation at a specific site of LIN-40 (the C. elegans homolog of MTA), a core NuRD component.
Genetic and biochemical analyses revealed that dephosphorylation of LIN-40/MTA promotes its interaction with the transcription factor DVE-1, which is essential for activating mitochondrial repair genes and chromatin remodeling, ultimately contributing to lifespan extension.
Moreover, they identified the kinase PMK-3 and phosphatase GSP-2 as key upstream regulators of LIN-40 phosphorylation.
This study reveals the functional and physiological significance of LIN-40/MTA dephosphorylation under mitochondrial stress, suggesting that novel mechanisms NuRD sense and respond to mitochondrial stress to promote longevity. It provides an important molecular basis for an in-depth understanding of mitochondria-to-nucleus signaling and aging regulation.
More information: Jun Zhou et al, Mitochondrial stress orchestrates chromatin remodeling and longevity via phosphoregulation of the NuRD component LIN-40, Science China Life Sciences (2025).
Journal information: Science China Life Sciences
Provided by Chinese Academy of Sciences