Novel molecular capsules can impart chirality to large, rigid metal-containing dyes

Lisa Lock
scientific editor

Robert Egan
associate editor

In nature, the concept of chirality or "handedness" is fundamental to life itself, just as our left and right hands are mirror images that cannot be overlapped perfectly. Molecular handedness is crucial in biological systems—the same molecule with different chirality acts as a healthful or toxic compound. Living organisms excel at creating chiral cavities through molecular self-assembly, allowing proteins to bind and transform substrates with high selectivity.
Despite intensive studies in synthetic chemistry, replicating such versatile chiral cavities artificially remains challenging, especially when using a self-assembly approach. Existing approaches often require a step-by-step synthesis of rigid chiral cavities, which limits their applicability.
One notable hurdle has been inducing chirality through weak interactions in highly symmetrical molecules, such as those used in advanced materials and catalysts. In particular, metal-containing dyes are difficult to "chiralize" because of their rigid and planar structures.
A research team led by Professor Michito Yoshizawa and Assistant Professor Yuya Tanaka from the Laboratory for Chemistry and Life Science at the Institute of Integrated Research, Institute of Science Tokyo (Science Tokyo), Japan, has developed an innovative approach to overcome these limitations.
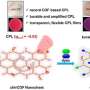
Their study, in the Journal of the American Chemical Society, reports the creation of chiral capsules that can impart strong chiral properties to inherently non-chiral metal-containing dyes.
The research team designed bent amphiphilic molecules, meaning that they had both water-loving and water-repelling parts. These were derived from 1,1'-binaphthyl-2,2'-diol or BINOL, a well-known chiral aromatic component. When dissolved in water, the molecules spontaneously self-assemble into spherical chiral capsules approximately 3 nanometers in diameter.
Unlike previous systems, these capsules create closed yet flexible chiral cavities that can adaptively encapsulate various metal-containing dyes, including metalloporphyrins, metallophthalocyanines, and metallonorcorroles.
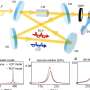
After optical experimental analyses, the researchers found that encapsulated dyes exhibit strong chiral activity, interacting effectively with polarized light at a level previously impossible to achieve without direct chemical modifications.
"To the best of our knowledge, the present capsules are the first molecular tools to induce chiral properties in common metal-containing dyes by simple encapsulation," say Tanaka and Yoshizawa. The team successfully demonstrated chirality induction in several types of metal-containing dyes, including metallophthalocyanines that are notoriously difficult to chiralize because of their relatively rigid and completely planar structures.
Moreover, the researchers discovered that the chiral properties of the encapsulated dyes can be tuned using thermal stimuli. By applying controlled heating, they could irreversibly adjust the intensity of the induced chirality, depending on the dyes. This thermal responsiveness transforms the capsule into a sophisticated nanotool that enables precise control over important molecular properties.
Overall, the present technology could prove useful in fields ranging from fundamental chemistry to applied science, where precise control over molecular chirality is essential. "Our new method allows for the introduction of chiral functionality into metal-containing dyes without procedures for multistep synthesis and complex separation. It holds great promise for the development of advanced photo-functional materials and asymmetric catalysts," conclude Tanaka and Yoshizawa.
More information: Yoshihisa Hashimoto et al, Chiral Aromatic Micelles as Chiroptical Host Tools for Large Metallodyes in Water, Journal of the American Chemical Society (2025).
Journal information: Journal of the American Chemical Society
Provided by Institute of Science Tokyo