Room-temperature synthesis produces hollow nanodome catalyst, slashing fuel cell costs and extending life

Sadie Harley
scientific editor

Robert Egan
associate editor

Hydrogen fuel cells, which produce electricity with high efficiency and zero greenhouse gas emissions, are gaining attention as a next-generation clean energy technology. However, their commercialization has been limited by performance degradation during prolonged operation and the high cost of catalyst replacement.
These issues stem from the instability of conventional catalysts, which suffer from metal dissolution and particle agglomeration over time, reducing reaction efficiency. To address this, the development of durable, high-performance catalysts that can be produced at low cost has become a critical research goal.
A joint research team led by Dr. Sung Jong Yoo at the Center for Hydrogen and Fuel Cells of the Korea Institute of Science and Technology (KIST), Professor Dong Won Chun of POSTECH, Professor Yongsoo Yang of KAIST, and Professor Haneul Jin of Dongguk University has developed a new catalyst technology that enables the synthesis of highly active and durable catalyst at room temperature using a simple ultrasound-assisted method.
The paper is in the journal Advanced Materials.
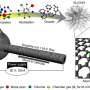
The newly developed catalyst features platinum and nickel precisely arranged into nanoscale domes with a hollow structure. This design increases the reactive surface area while minimizing catalyst loss, resulting in significantly improved performance.

Traditionally, creating such precise nanostructures required complex processes at temperatures exceeding 600掳C. In contrast, the new method enables atomic rearrangement using just a one-step ultrasound process at room temperature.
The researchers employed an ultrasonic device similar to those used in eyeglass cleaning to naturally guide metal atoms into ordered structures, significantly simplifying the manufacturing process and lowering production costs with enhanced activity and durability.
In half-cell tests designed to measure the intrinsic catalytic activity, the new catalyst showed about seven times higher mass activity compared to commercial catalysts. Even in full-cell tests under practical fuel cell conditions, it maintained a notable lead with about five times higher mass activity.
In durability evaluations conducted according to U.S. Department of Energy (DOE) protocols, the catalyst remained stable for over 42,000 hours鈥攎ore than 4.2 times the lifespan of currently available commercial catalysts. This breakthrough is expected to reduce replacement intervals and maintenance costs in large-scale fuel cell systems used in trucks, buses, ships, and power plants.

Catalysts account for over 30% of the total manufacturing cost of fuel cell systems. By extending catalyst lifespan and boosting performance, the new technology significantly enhances the economic viability of hydrogen fuel cells.
The team is currently exploring various transition metal combinations to further expand the technology, while also conducting fuel cell stack-level evaluations and demonstration studies for automotive applications.
Dr. Yoo of KIST stated, "Our catalyst features a unique dome-shaped nanostructure with precisely arranged atoms, resulting in substantial improvements in both activity and durability. Because the process works at room temperature, we believe this technology can play a meaningful role in advancing the commercialization of hydrogen fuel cells and achieving carbon neutrality."
More information: Eungjun Lee et al, Suppressing Metal Dissolution in Multi鈥怗rained Catalysts Through Intragrain Atomic Ordering for Stable Fuel Cells, Advanced Materials (2025).
Journal information: Advanced Materials