Scientists design superdiamonds with theoretically predicted hexagonal crystal structure

Sanjukta Mondal
contributing writer

Sadie Harley
scientific editor

Robert Egan
associate editor

The brilliantly shiny diamond is more than just pretty; it's one of the hardest minerals on Earth, with a name derived from the Greek word adámas, meaning unbreakable. Scientists have now engineered a harder form of diamond known as bulk hexagonal diamond (HD)—a crystalline structure that has been theorized for over half a century to have physical properties superior to those of conventional diamond.
In a in Nature, researchers from China synthesized bulk hexagonal diamond, ranging from 100-µm-sized to mm-sized, with a highly ordered structure by compressing and heating high-quality graphite single crystals under pressure conditions as uniform as possible.
The designed material, which was recoverable under ambient conditions, unveiled the previously elusive structural world of HD, opening new avenues for exploring its potential as a technologically superior material.
Apart from being a dazzling element in jewelry, diamonds, due to their unmatched chemical and physical properties, are sought after in a wide range of applications, including biosensors, quantum computing, and industrial processes as superabrasives and drilling bits.
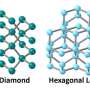
These properties arise from its unique cubic atomic structure, where each carbon atom is bonded to four other carbon atoms via strong sp3 bonds, giving rise to a rigid tetrahedral network.
These structures then form honeycomb layers that stack on top of each other to build cubic diamond crystals. The natural cleavage planes created by these layered structures is also the reason behind diamond's strength limit, something that research suggests can be rectified by selectively strengthening and shortening the interlayer bonds. This change, however, would transform the cubic diamond into a hexagonal crystal symmetry.

Similar transformations aren't unseen in nature, for instance, HD, also known as lonsdaleite, is found in Canyon Diablo meteorite, where it was named.
A 2022 study suggested that the high temperatures and shock compressions created during the meteor impact turn graphite in the meteorite into HD.
Scientists have attempted to emulate similar explosive and high-pressure conditions in laboratories to produce artificial lonsdaleite but most samples are too small and often impure, making it difficult to isolate and study the true properties of HD.
Now, the researchers have successfully synthesized a highly ordered and nearly pure bulk HD, which was recovered intact under ambient conditions. Starting from single-crystal hexagonal graphite, they created a controlled quasi-hydrostatic environment—applying pressure as uniformly as possible to minimize stress variations—using both a diamond anvil cell (DAC) and a large-volume multi-anvil press.
The bulk sample consisted of a threefold intergrowth of densely packed crystals, each approximately 100 nanometers in size, primarily composed of HD with minor imperfections, including traces of cubic diamond.

Investigating the molecular-level mechanisms via spectroscopy revealed a complete conversion of sp2 bonds into sp3 bonds, indicating a direct crystallographic transformation from graphite to diamond.
They also found that the interlayer bonds of HD were stronger and shorter than those in conventional cubic diamond, which was reflected in the Vickers hardness test—which measures a material's resistance to plastic deformation—where the values for HD were slightly higher.
The researchers note that this study unambiguously demonstrates the existence of HD as a bona fide phase of carbon with superior hardness, similar to that of cubic diamond. They believe that experimenting with levels of graphite precursor purification and fine-tuning the pressure–temperature conditions can lead to the creation of even higher-quality HD with superior properties.
Written for you by our author , edited by , and fact-checked and reviewed by —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Liuxiang Yang et al, Synthesis of bulk hexagonal diamond, Nature (2025).
Journal information: Nature
© 2025 Science X Network