Two solutions unlock safer RNA therapies for inflammatory diseases

Gaby Clark
scientific editor

Robert Egan
associate editor

Lipid nanoparticles (LNPs) are tiny fat bubbles that are used to deliver medicines, genes, and RNA into cells. However, in some cases LNPs can cause harmful inflammation as a result of the process of RNA delivery.
Now, two new solutions can help alleviate inflammation while still getting RNA where it needs to be in the cell. One discovery found that inflammation could be reduced with the addition of a unique biodegradable lipid to the treatment; another solution identified a common drug, called thiodigalactoside (TG), which blocked inflammation when added to the LNP. Today's features this research from the Perelman School of Medicine at the University of Pennsylvania.
"For patients with inflammatory diseases like ARDS, heart attack, or stroke, our solutions– a new lipid and a galectin-blocking drug—make RNA therapies safer," said study co-author Jacob Brenner, MD, Ph.D., an assistant professor of Pulmonary, Allergy and Critical Care.
Patching holes, safer RNA delivery
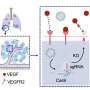
LNPs enter cells with the help of endosomes, tiny sacs which help direct material entering cells to the right places. The research team found that when LNPs are delivering their "load" of RNA, the endosomes rupture like a burst balloon, allowing harmful substances to leak out and spark immune responses. These holes are detected by proteins called galectins, which drive inflammation.
Scientists found that adding a special fat molecule, 4A3-SC8, makes smaller holes that the cell can quickly patch up, reducing inflammation while keeping RNA delivery effective. In other words, the endosome springs a leak and the fat molecule can fix it.
They also discovered that a readily available but uncommon drug called thiodigalactoside (TG) can block inflammation when added to LNPs. TG is normally used to treat inflammation and cancer.
These strategies proved transformative in a mouse model of acute respiratory distress syndrome (ARDS), a common disease where fluid builds up in the lungs and oxygen levels drop to dangerous levels. Using either new treatment, the team delivered mRNA to treat the ARDS, which dramatically reduced lung inflammation and tissue damage without the harmful side effects typically caused by LNPs.
A step forward for RNA
"By designing LNPs that cause less damage and block inflammation pathways, we can expand RNA treatments to conditions like ARDS, heart attack, and stroke, where inflammation is a major challenge," Brenner said.
He points out that these findings don't mean COVID-19 vaccine LNPs cause harmful inflammation. "Vaccines rely on LNPs to stimulate the immune system throughout the body, which is key to their success, but this immune activation can worsen conditions like stroke or ARDS when LNPs are used as treatments," Brenner explained. "Our study shows how to make LNPs safer for such diseases by reducing unwanted inflammation."
The findings mark a significant step forward for RNA therapeutics, which have shown promise in treating cancers, genetic disorders, and now inflammatory diseases.
"This represents meaningful progress for RNA-based therapeutics," said first author Serena Omo-Lamai, a Ph.D. student researcher. "Our approaches could make LNPs safer and more versatile, opening doors to treat inflammatory diseases that were previously out of reach."
More information: Omo-Lamai, S., Wang, Y., Patel, M.N. et al. Limiting endosomal damage sensing reduces inflammation triggered by lipid nanoparticle endosomal escape. Nature Nanotechnology (2025).
Journal information: Nature Nanotechnology