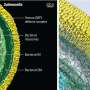
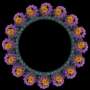
Credit: Yale University
The Yale labs of Craig Roy and Jun Liu have harnessed the power of cryo-EM to solve a 30-year mystery of how the Legionella bacteria works. The findings represent the next steps in the search for new therapeutic drugs to tackle the severe form of pneumonia.
Early in his postdoc studies, Roy wanted to understand why a single protein was essential for the bacteria Legionella to cause disease.
Fast forward more than 30 years, and now alongside collaborative partner Jun Liu, the Yale professors are using next-generation research technology to reveal a level of 3D precision they could only have dreamed of.
The team's recent breakthrough has revealed the tiny biological nano-machine used by Legionella to infect our host cells—and with it a better understanding of Legionnaires' disease, a serious pneumonia that affects about 10,000 people in the United States annually.
For Samira Heydari and Jian Yue, postdoctoral associates in the Liu Lab and co-first authors of the findings in the Proceedings of the National Academy of Sciences, using cryo-electron microscopy (cryo-EM) has propelled the scientists to catch the bacteria quite literally "in the act."
Yale scholars Samira Heydari and Jian Yue explain their discovery. Credit: Yale University
Until recently, light and electron microscopes have limited scientists to studying biological samples at comparably lower resolutions. The arrival of cryo-EM has taken the fields of structural and cell biology to the next exciting level, allowing scientists to freeze whole live cells in their search for knowledge underlying human disease.
"I've been looking for the answer for 33 years," said Roy, who is the Waldemar Von Zedtwitz Professor of Microbial Pathogenesis and of Immunobiology.
"Back in 1992 we only knew about just one protein—called DotA—that coded for this machinery. It took time and the emergence of new tools, alongside new expertise, to figure out how something in the bacteria's membrane was communicating with our host cells."
The scholars figured out that DotA wasn't acting alone and was actually one component of a very complex machine. But less than a decade ago, before the advent of cryo-EM and the "resolution revolution," pictures of the machine remained hazy.
"We were only able to see a structure like a cartoon pictograph, without being able to identify any individual components," recalls Roy.
Two things happened to change the game.
In 2017, Yale's first cryo-electron microscope arrived at Yale's West Campus. Around the same time, Roy was contacted by Jun Liu, then a faculty member at the University of Texas Houston Medical School. He was interested in helping to solve the Legionella puzzle.
"The greatest technology doesn't happen in a vacuum. It's driven by scientists like Jun and his team, adapting new technology and applying it to very difficult questions," said Roy, who was a founding member of Yale's Department of Microbial Pathogenesis back in 1998.
"Proximity helps too," says Roy about what happened next. Jun Liu joined the Yale faculty in short order, sending their discovery process into overdrive. Liu is now a Professor of Microbial Pathogenesis and member of the Yale Microbial Sciences Institute, also at the West Campus.
The scholars moved away from studying the individual DotA protein in pursuit of a better understanding of what was being delivered by the nano-machine and how it was controlling host-cell function.
Yue and Heydari set to the hard task of identifying and piecing together more than 76,000 particles to bring to life the nanomachine's full structure. Undeterred by high failure rates, the scholars were finally able to identify 20 different Dot proteins responsible for the nanomachine structure and its functions.
"We needed the power of the cryo-EM to be able to manipulate the bacterial cells so we could finally reveal the action," said Heydari of the discovery.
Doing so enabled the scholars to illuminate their data in high-definition 3D film, showing the nano-machine shifting its gear assembly from closed to an open channel used by Legionella to deliver bacterial "effector" proteins into host cells to hijack various cell functions.
"To now be able to visualize the single protein I began working on some 30 years ago as one of the critical elements of the channel—it's a dream come true," continued Roy.
With an altogether more accurate picture, a deeper dive into the real time dynamics of the nanomachine is within reach. The mechanical channel is known to carry over 300 diverse proteins, suggesting a broader use and value for clinical applications.
"Big discoveries don't happen overnight," enthuses Roy about what's to come. "They come from understanding numerous basic principles."
"Does the channel work like a sewing machine needle to deliver its cargo, or is it a trigger-like mechanism that embeds in our host cells?" The team are determined to get to the bottom of how this works.
Important for fighting diseases like Legionnaires' directly, the scientists say the findings may have broader impact. They aim, for example, to learn how to manipulate the bacteria's ability to transfer antibiotic-resistant genes.
The arrival of novel technology is providing new impetus in similar collaborations across Yale that are expected to have wide-reaching impact.
"Harnessing the power of this new technology is no longer a question for one scientist in a lab," concludes Jun Liu. "We need to communicate what cryo-EM can do across Yale to a broad group of scientists.
"Having the technology here is a vital step towards developing future medications."
More information: Jian Yue et al, In situ structures of theLegionellaDot/Icm T4SS identify the DotA–IcmX complex as the gatekeeper for effector translocation, Proceedings of the National Academy of Sciences (2025).
Journal information: Proceedings of the National Academy of Sciences
Provided by Yale University