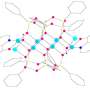
Traditional coordination complexes of olefins with metals (left) and the newly-discovered olefin coordination complexes with boron (right). Credit: Rian Dewhurst
When it comes to eliminating toxic and expensive heavy metals in the chemical industry, a new study from the University of Würzburg points the way forward.
The team led by chemistry professor Holger Braunschweig at the University of Würzburg is investigating the "metal-mimetic" properties of main group elements such as boron. They have shown that under certain conditions, boron can mimic the reaction behavior of metals without being toxic or as expensive as metals.
The article in Nature Chemistry shows that boron can also form so-called π complexes with olefins, which are similar in their properties and behavior to the complexes of transition metals with olefins. The latter compounds are intermediates in many large-scale catalytic processes in industry.
"Our discovery opens up a whole new area of the periodic table for π coordination chemistry—including the possibility of using main group elements as industrial catalysts for functionalization reactions of unsaturated hydrocarbons," says Braunschweig.
The boron–olefin π complexes were synthesized by postdocs Dr. Maximilian Michel and Dr. Marco Weber. The Würzburg chemists hope that their discovery will inspire other researchers to push the boundaries of main group chemistry further and find additional applications for boron or other main group elements.
"In the long term, our main goal is to replace toxic and costly heavy metals in industrial processes with main group elements," says Braunschweig.
Next, the team wants to modify the boron–olefin π complexes so that their behavior is even more similar to that of the known metal complexes.
More information: Maximilian Michel et al, Olefin π-coordination chemistry at low-oxidation-state boron, Nature Chemistry (2025).
Journal information: Nature Chemistry
Provided by Julius Maximilian University of Würzburg