Researchers capture new antibiotic resistance mechanisms with trace amounts of DNA

Stephanie Baum
scientific editor

Robert Egan
associate editor

Scientists from the University of Illinois Urbana-Champaign have developed a method to isolate genes from amounts of microbial DNA so tiny that it would take 20,000 samples to weigh as much as a single grain of sugar.
In a appearing in mSystems, the researchers discovered previously unknown antibiotic resistance genes in bacterial DNA isolated from human stool and from fish tanks at Chicago's Shedd Aquarium.
"With antibiotic resistance on the rise, it's more important than ever to understand the full diversity of mechanisms bacteria may be using to inactivate or avoid antibiotics," said Terence Crofts, assistant professor in the Department of Animal Sciences, part of the College of Agricultural, Consumer and Environmental Sciences at Illinois. "If we can get a clearer view of the antibiotic resistance genes that exist in the environment, that will give biomedical researchers a chance to look out for them in the clinic and potentially design more effective drugs."
Crofts developed the method, known as , to improve a microbiology tool known as a functional metagenomic library, which enables researchers to capture bacterial genes from the environment. The method allows researchers to collect soil, stool, or other environmental samples and screen for the presence of potentially new microbial genes without having to culture the microbes or sequence their genomes. But METa assembly requires 100 times less DNA than standard functional metagenomic libraries, which is useful where microbes are scarce or when researchers can't take large samples.
"We used to take the DNA out of the bacteria and just sequence it, but there are so many new genes in those environments that our sequencing ability has far outstripped our ability to actually guess at the functions of those genes," Crofts said. "A lot of these genes have unknown functions, so functional metagenomic screens are a way to get around that problem."
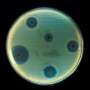
Instead of sequencing the DNA, the researchers use an enzyme to chop it into gene-sized pieces, which they then introduce to E. coli bacteria in the lab. E. coli, which is easier to grow in lab settings than many other microbes, incorporates the foreign DNA into its genetic machinery and begins to take on its traits. Crofts says antibiotic resistance traits are especially well-suited for study using functional metagenomic libraries because they're usually controlled by a single gene, and it's easy to tell whether bacteria have it or not.
"If E. coli has a resistance gene, it can survive an antibiotic. If it doesn't, it dies," he said. "We might have 10 million E. coli cells in a petri dish with 10 million unique random chunks of environmental DNA. If we expose it to a particular antibiotic and only 10 colonies survive, we know those 10 had a resistance gene. Then it becomes very easy to take those colonies and sequence the chunk of DNA they grabbed from that environmental sample."
Even if the sequencing result turns up genes for unknown proteins, the researchers know that they have a role in antibiotic resistance and can immediately drill down to study their mechanisms.
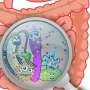
Crofts and his team tested the method on a sample of water from a large tank at Chicago's Shedd Aquarium, where microbes are far less populous than in other environments like soil. They also tested a tiny sample from a product that usually teems with bacteria: human fecal matter.
"Because aquatic samples are usually less dense with microbes, you usually can't get as much DNA out of them, but we showed that we could still make good libraries from the aquarium sample," Crofts said. "It's also significant that we could make a functional metagenomic library from just a swab sample of fecal matter. That could be useful for clinical settings."
The team didn't just make libraries from these samples, they made new discoveries about how microbes resist antibiotics. For example, in tetracycline-resistant sequences from Shedd Aquarium, the researchers identified new types of efflux pumps—protein channels that pump substances across the cell membrane—that remove tetracycline from cells.
Interestingly, some of the E. coli colonies from the human fecal sample resisted a group of antibiotics known as streptothricins. These were tested in the 1940s, but were never brought to market due to kidney toxicity in mammals. But with so much resistance in the current antibiotic landscape, Crofts says biomedical researchers are looking into streptothricin again (in less toxic forms).
"We found what looks like an entirely new family of streptothricin resistance proteins in our sequences," he said. "Streptothricin is being brought up as this potentially clinically useful antibiotic, but we should really be trying to find out what resistance is already out there in the environment. And instead of making traditional streptothricins less toxic, maybe we should make a next-generation analog that can beat the antibiotic resistance mechanisms that may already exist in nature."
Crofts plans to deploy his METa assembly method to agricultural systems—sampling soil and from "nose to tail" in livestock—since antibiotic resistance not only occurs on the farm, it often originates on the farm.
"We produce a lot of antibiotics by culturing soil bacteria that make antibiotics as weapons to fight other bacteria. So, soils therefore have a very rich diversity of antibiotic resistance genes," Crofts said. "Agriculture puts all these mammals in close association with this reservoir of antibiotic resistance genes in the soil. Since we're giving these animals large amounts of antibiotics, it becomes a very ripe environment for resistance to develop and jump into bacteria that can impact our own health."
More information: mSystems (2025).
Journal information: mSystems
Provided by University of Illinois at Urbana-Champaign