Metallic nanocatalysts: What really happens during catalysis

Stephanie Baum
scientific editor

Robert Egan
associate editor

Using a combination of spectromicroscopy at BESSY II and microscopic analyses at DESY's NanoLab, a team has gained new insights into the chemical behavior of nanocatalysts during catalysis.
The research is in the journal ACS Nano.
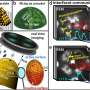
The nanoparticles consisted of a platinum core with a rhodium shell. This configuration allows a better understanding of structural changes in, for example, rhodium–platinum catalysts for emission control. The results show that under typical catalytic conditions, some of the rhodium in the shell can diffuse into the interior of the nanoparticles. However, most of it remains on the surface and oxidizes. This process is strongly dependent on the surface orientation of the nanoparticle facets.
Nanoparticles measure less than one ten-thousandth of a millimeter in diameter and have enormous surface areas in relation to their mass. This makes them attractive as catalysts: metallic nanoparticles can facilitate chemical conversions, whether for environmental protection, industrial synthesis or the production of (sustainable) fuels from CO2 and hydrogen.
Platinum core with rhodium shell
Platinum (Pt) is one of the best-known metal catalysts and is used in heterogeneous gas phase catalysis for emission control, for example to convert toxic carbon monoxide in car exhaust gases from combustion engines into non-toxic CO2.
"Mixing platinum particles with the element rhodium (Rh) can further increase efficiency," says Jagrati Dwivedi, first author of the publication. The location of the two elements plays an important role in this process. So-called core-shell nanoparticles with a platinum core and an extremely thin rhodium shell can help in the search for the optimal element distribution that can extend the lifetime of the nanoparticles.
Experiments at BESSY II and DESY NanoLab
Until now, however, little was known about how the chemical composition of a catalyst's surface changes during operation. A team led by Dr. Thomas F. Keller, head of the microscopy group at DESY NanoLab, has now investigated such crystalline Pt-Rh nanoparticles at BESSY II and gained new insights into the changes at the facets of the polyhedral nanoparticles.
The nanoparticles were first characterized and marked in their vicinity using scanning electron microscopy and atomic force microscopy at DESY NanoLab. These markers were then used to analyze the same nanoparticles spectroscopically and image them microscopically simultaneously using X-ray light on a special instrument at BESSY II.
The SMART instrument at the Fritz Haber Institute of the Max Planck Society enables X-ray photoemission electron microscopy (XPEEM) in a microscope mode. This makes it possible to distinguish individual elements with high spatial resolution, enabling the observation of chemical processes at near-surface atomic layers.
"The instrument allows the chemical analysis of individual elements with a resolution of 5–10 nanometers, which is unique," says Keller.
The investigation has shown that rhodium can partially diffuse into the platinum cores during catalysis: both elements are miscible at the typical operating temperatures of the catalyst. The mixing is enhanced in a reducing environment (H2) and slowed down in an oxidizing environment (O2) without reversing the net flow of rhodium into platinum.
"At higher temperatures, this process even increases significantly," explains Keller.
Different reaction rates
The reaction rates also depend on the orientation of the nanoparticles' facets.
"They are particularly high on certain facets," emphasizes Jagrati Dwivedi. "Our facet-resolved study shows that rhodium oxidation is highest on facets with many atomic steps, where the atoms are most easily bound."
This detailed analysis of the oxidation behavior will contribute to the further optimization of such nanocatalysts, which can undergo irreversible changes during use.
More information: Jagrati Dwivedi et al, Spectro-Microscopy of Individual Pt–Rh Core–Shell Nanoparticles during Competing Oxidation and Alloying, ACS Nano (2025).
Journal information: ACS Nano
Provided by Helmholtz Association of German Research Centres