Catalyst evolution reveals the unsung heroes in industrial ammonia production

Gaby Clark
scientific editor

Robert Egan
associate editor

Researchers at the Fritz Haber Institute of the Max Planck Society, in collaboration with the Max Planck Institute of Chemical Energy Conversion and Clariant have unveiled new insights into the complex catalyst systems used in industrial ammonia production. By examining the structural evolution of these catalysts, the study highlights the critical role of promoters in enhancing performance and stability.
The Haber-Bosch process, a cornerstone of industrial ammonia production, has remained largely unchanged for over a century. However, researchers at the Departments of Inorganic Chemistry and Interface Science of the Fritz Haber Institute, the Max Planck Institute for Chemical Energy Conversion, and Clariant have made significant strides in the mechanistic understanding of the highly complex industrial catalyst that drives this process.
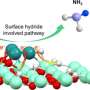
By using advanced characterization techniques like operando scanning electron microscopy and near-ambient pressure X-ray photoelectron spectroscopy, the team has decoded the complex interactions within multi-promoted ammonia synthesis catalysts.
Prof. Thomas Lunkenbein, the corresponding author, stated, "Our research provides a deeper understanding of the catalyst's inner workings, revealing how promoters and structural transformations contribute to its efficiency and stability. This knowledge is crucial for developing next-generation catalysts that are both more effective and sustainable."
The findings are in the journal Nature Communications.
The study reveals that the activation phase is crucial for forming the active catalyst configuration. During this phase, the interplay of various promoter phases facilitates the transformation of the catalyst's structure into a porous entity with a special surface coverage paving the way for its enhanced performance and longevity.
Promoters: The unsung heroes
Promoters, including potassium, calcium, and aluminum oxides, are vital in stabilizing the catalyst's structure and boosting its activity. These elements work together to create cement-like phases—an important ingredient for robust and efficient catalyst capable of sustaining ammonia synthesis over extended periods. In addition, ammonia K—a special form of highly dispersed K+ species—was found to be the pacemaker of the catalytic reaction.
The research highlights the importance of the catalyst's hierarchical porous structure, which is stabilized by mineral phases. This architecture not only enhances the catalyst's durability but also its resistance to deactivation, ensuring consistent performance in industrial settings.
This study sheds light on the intricate dynamics of ammonia synthesis catalysts, offering valuable insights that could pave the way for future innovations in industrial chemistry, including the strong need to consider the dynamic nature of active catalytic surfaces while at work.
By understanding the role of promoters and the critical role of the activation process, researchers can develop more efficient and sustainable catalysts for ammonia production. We acknowledge the expertise and input of Prof. Dr. Robert Schlögl, who, together with a team of excellent scientists, led to this important scientific contribution.
More information: Luis Sandoval-DÃaz et al, Decoding technical multi-promoted ammonia synthesis catalysts, Nature Communications (2025).
Journal information: Nature Communications
Provided by Max Planck Society