How egg cells control the timing of cell division

Sadie Harley
scientific editor

Robert Egan
associate editor

A protein known as MPS1 helps to ensure that a key process during cell division of oocytes—unfertilized egg cells—occurs in a timely manner, RIKEN biologists have discovered. This finding in mice may have implications for pregnancy loss and congenital diseases in people.
The is published in The EMBO Journal.
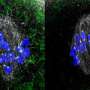
At any particular moment, millions of cells in our bodies are undergoing division. During cell division, cellular machinery known as spindles, which are made up of microtubules, divide up the genetic material in a cell.
For normal cells, this process is orchestrated by two specialized structures known as centrosomes. But since oocytes lack centrosomes, they have to rely on another method for assembling spindles.
Microtubules attach to protein "handles" on spindles called kinetochores. There is a high risk that incorrect attachment will occur if it happens before the spindles have had a chance to form two distinct poles. Incorrect attachment can have serious consequences, including miscarriage and the development of disorders such as Down syndrome.
However, little is known about the mechanism by which oocytes control the timing of spindle bipolarization relative to kinetochore-microtubule attachment.
Now, in a mouse experiment, Tomoya Kitajima of the RIKEN Center for Biosystems Dynamics Research and co-workers have found that MPS1 plays a key role in ensuring that spindle bipolarization occurs before kinetochores attach to microtubules.
They found that spindle bipolarization is often delayed in oocytes in which MPS1 had been inhibited, and that this delay resulted in incorrect attachments.
The finding adds to MPS1's repertoire of functions. "MPS1 had been known to have another function as an activator of the spindle assembly checkpoint," says Kitajima. "The finding indicates a new function of MPS1, namely that of promoting timely spindle bipolarization."
Based on this finding, the team proposed a two-stage model for spindle assembly. In this model, kinetochores first promote spindle bipolarization in unstably attached microtubules and then stabilize microtubule attachment in the bipolarized spindle. This order reduces the risk of errors occurring.
If MPS1 plays a similar role in humans, then the discovery could find application in treatments for infertility. "Human oocytes often show delay in spindle bipolarization, which leads to eggs with an abnormal number of chromosomes, the leading cause of pregnancy loss and congenital disease," says Kitajima.
"Functionality of MPS1 in human oocytes may be a key to predict and control oocyte quality in assisted reproductive technologies."
Kitajima is now exploring whether MPS1 could be used to enhance artificial kinetochores that his team had developed previously.
More information: Shuhei Yoshida et al, MPS1 promotes timely spindle bipolarization to prevent kinetochore-microtubule attachment errors in oocytes, The EMBO Journal (2025).
Journal information: EMBO Journal
Provided by RIKEN