'Inert' scandium unlocked as a powerful blue-light photocatalyst

Gaby Clark
scientific editor

Robert Egan
associate editor

Scientists at the University of Chemistry and Technology, Prague (UCT Prague) have revealed a new catalytic role for scandium, an element whose salts were long considered to be redox stable, i.e. not participating in redox reactions. in Nature Communications, their research shows a simple scandium salt can act as a potent photocatalyst, using blue light to drive important organic oxidation reactions.
Led by Professor Radek Cibulka, the team demonstrated that scandium triflate (Sc(OTf)₃) efficiently powers key chemical transformations, including the aerobic oxidation of compounds like toluene into valuable acids using oxygen from the air and the direct C-C bond formation needed to attach cyano groups to aromatic rings—a vital step in drug discovery.
The discovery overturns the long-held belief that scandium is "redox-inert" and unsuited for photocatalysis, providing an alternative to traditional catalysts based on expensive, rare metals like iridium and ruthenium. "The discovery was completely unexpected," says Prof. Cibulka. "It points to a completely new application for scandium, a relatively abundant but neglected metal."
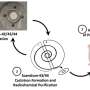
The team's research shows that scandium's strong affinity for oxygen is key. The scandium ion forms a complex with molecular oxygen, and it is this scandium-oxygen complex that absorbs the light. In its energized state, it can strip an electron from an organic molecule, initiating the chemical reaction. This crucial scandium-superoxide intermediate was directly observed, confirming the novel mechanism.
The work, part of the Eco&Stor project, was a collaboration between UCT Prague, the J. Heyrovský Institute of Â鶹ÒùÔºical Chemistry, and international partners.
More information: Amal Hassan Tolba et al, Redox-innocent scandium(III) as the sole catalyst in visible light photooxidations, Nature Communications (2025).
Journal information: Nature Communications
Provided by University of Chemistry and Technology Prague