Atomic 'CT scan' reveals how gallium boosts fuel cell catalyst durability

Gaby Clark
scientific editor

Robert Egan
associate editor

Hydrogen fuel cell vehicles have long been hailed as the future of clean mobility: cars that emit nothing but water while delivering high efficiency and power density. Yet a stubborn obstacle remains. The heart of the fuel cell, the platinum-based catalyst, is both expensive and prone to degradation. Over time, the catalyst deteriorates during operation, forcing frequent replacements and keeping hydrogen vehicles costly.
Understanding why and how these catalysts degrade at the atomic level is a longstanding challenge in catalysis research. Without this knowledge, designing truly durable and affordable fuel cells for mass adoption remains out of reach.
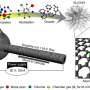
Now, a team led by Professor Yongsoo Yang of the Department of Â鶹ÒùÔºics at KAIST (Korea Advanced Institute of Science and Technology), in collaboration with Professor Eun-Ae Cho of KAIST's Department of Materials Science and Engineering, researchers at Stanford University and the Lawrence Berkeley National Laboratory, has successfully tracked the three-dimensional change of individual atoms inside fuel cell catalysts during thousands of operating cycles. The results provide unprecedented insight into the atomic-scale degradation mechanisms of platinum-nickel (PtNi) catalysts, and demonstrate how gallium (Ga) doping dramatically improves both their performance and durability.
The study is in the journal Nature Communications.
A new atomic 'CT scan' for catalysts
To achieve this breakthrough, the team utilized a neural network-assisted atomic electron tomography (AET) technique. Much like a CT scan in a hospital reconstructs the inside of the human body from X-ray images, AET determines the positions of thousands of atoms inside nanomaterials from high-resolution electron microscopy images taken at many different angles. By combining these reconstructions with advanced AI-based data correction, the researchers were able to map the exact 3D coordinates and chemical identity of every atom in the nanoparticle catalysts.
This allowed them to directly observe—at single-atom resolution—how the catalysts changed in structure, chemical composition, and internal strain as they were cycled thousands of times under fuel cell operating conditions.
Why gallium makes a difference
The researchers compared conventional PtNi catalysts with Ga-doped PtNi catalysts. The results revealed:
- Shape stability: While undoped PtNi particles gradually lost their advantageous octahedral shape and became more spherical (i.e., the fraction of highly active {111} facets has been reduced), Ga-doped particles retained their octahedral shape even after 12,000 cycles.
- Chemical stability: In PtNi catalysts, nickel atoms leached from both the surface and subsurface regions, driving structural instability. In Ga-doped catalysts, surface nickel was largely preserved, preventing collapse of the structure.
- Strain preservation: The compressive strain in PtNi particles, crucial for optimizing oxygen reduction activity, relaxed substantially over time. In contrast, Ga-doped particles maintained near-optimal strain.
- Catalytic performance: By integrating these factors, the researchers showed that while undoped PtNi catalysts lost ~17% of their oxygen reduction activity after 12,000 cycles, Ga-doped PtNi catalysts lost only ~4% and maintained superior activity throughout.
Dr. Yang, who led the research, explained the significance of the results: "These results represent the first time the true 3D atomic-scale degradation dynamics of fuel cell catalysts have been directly visualized. Our findings not only reveal why gallium doping works, but also establish a powerful framework for rationally designing durable, high-efficiency catalysts."
Implications for a hydrogen-powered future
The study demonstrates that neural network-assisted AET can reveal how nanomaterials evolve during real operating conditions, overcoming the limitations of traditional 2D imaging and ensemble-averaged methods. Beyond PtNi catalysts, the technique can be applied to a wide range of nanomaterials and catalytic systems, helping to design the next generation of energy materials with atomic precision.
For the hydrogen economy, this means that more durable catalysts could extend the lifetime of fuel cells, lower replacement costs, and accelerate the widespread adoption of hydrogen-powered vehicles and clean energy technologies.
More information: Chaehwa Jeong et al, Atomic-scale 3D structural dynamics and functional degradation of Pt alloy nanocatalysts during the oxygen reduction reaction, Nature Communications (2025).
Journal information: Nature Communications