Scientists uncover how cellular receptors trigger inflammation and sensory changes

Stephanie Baum
scientific editor

Robert Egan
associate editor

In two new studies, scientists at Oregon Health & Science University have uncovered detailed blueprints of how certain molecular "gates" in human cells work—findings that could open doors to new treatments for conditions ranging from certain cancers and brain diseases to hearing loss and atherosclerosis, or plaque build-up in the arteries.
The research comes from the lab of Steven E. Mansoor, M.D., Ph.D., associate professor of cardiovascular medicine, physiology and biochemistry in the OHSU School of Medicine and Knight Cardiovascular Institute. His lab studies a group of proteins known as P2X receptors, which sit on the surface of cells and detect ATP—a molecule best known as the body's energy source inside of cells.
When ATP leaks outside of cells, often as a sign of stress or damage, P2X receptors act like alarm bells, triggering responses related to inflammation, pain and sensory processing.
"Extracellular ATP is a universal danger signal," Mansoor said. "When it builds up outside cells, P2X receptors sense it and change how the cells respond. Understanding these receptors at the atomic level is key to designing drugs that can either calm them down or fine-tune their activity."
Mapping a receptor linked to inflammation
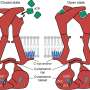
In a study in Nature Communications, researchers examined the molecular structure of the human P2X7 receptor, a protein linked to inflammatory diseases such as cancer, Alzheimer's and atherosclerosis. Despite years of effort, no drugs targeting P2X7 have reached the clinical market, partly because drugs that have worked well in animal models have not had the same success in humans.
Building on a by Mansoor and graduate student Adam Oken, where they determined how to turn the rat P2X7 receptor off, the team has now mapped how drugs turn off the human P2X7 receptor for the first time. They now know what makes the human receptor different from the receptor that is present in animal models. This is important for understanding how to better customize drugs to fit the binding pockets within the human receptor.
Using that information, the researchers, in collaboration with groups from around the world, designed a new compound referred to as UB-MBX-46. The compound complements the binding pocket in the human receptor, translating to a molecule that blocks the human receptor with high precision and strength.
"This is the first time we've visualized the human P2X7 receptor and really understood how it is different from others," Oken said. "With that knowledge, we can create a drug candidate that perfectly fits binding pockets within the human receptor, much like how a key fits in a lock. It gives us hope for developing therapies that have better chances to reach the clinic."
New insight into hearing-related receptor
A second study in Proceedings of the National Academy of Sciences examined the human P2X2 receptor, a protein in the same family as the P2X7 receptor, but is predominantly found in the cochlea, the hearing organ of the inner ear.
The P2X2 receptor is involved in hearing processes and in the ear's adaptation to loud noise. Certain genetic mutations of this receptor have been linked to hearing loss. Currently, there are no drugs that target this receptor effectively, and until now, scientists had limited insight into how it functions.
Led by Franka Westermann, a Ph.D. student at the University of Bonn, researchers at OHSU used cryo-electron microscopy—a powerful imaging method—to capture 3D structures of the human P2X2 receptor in two states: in a resting state and in a state bound to ATP but desensitized, meaning it's not active anymore.
The team discovered unique structural features and pinpointed areas where hearing-related mutations occur.
"Our findings show exactly how ATP binds and how the receptor changes on a molecular level after it gets activated," Westermann said. "It is very instructive to see how P2X2 is different than P2X7. This will help us be able to design molecules that are more specific to the receptor we want to control."
A foundation for future therapies
Together, the studies mark a leap forward in understanding how P2X receptors contribute to a wide range of diseases by triggering inflammation and sensory changes.
"This is foundational work," Mansoor said. "By uncovering these structures, we're laying the groundwork to develop selective molecules that could address a wide range of human diseases. I am actively taking my research program into this translational direction."
More information: Adam C. Oken et al, A polycyclic scaffold identified by structure-based drug design effectively inhibits the human P2X7 receptor, Nature Communications (2025).
Franka G. Westermann et al, Subtype-specific structural features of the hearing loss–associated human P2X2 receptor, Proceedings of the National Academy of Sciences (2025).
Journal information: Proceedings of the National Academy of Sciences , Nature Communications
Provided by Oregon Health & Science University