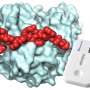
Crystal structure of calprotectin with zinc bound at the His₆ sites. (A) Overall structure of the calprotectin heterotetramer. Zn²⁺ ions are shown as gray spheres, Ca²⁺ ions as green spheres, and water molecules as red spheres. (B) Close-up of a Zn²⁺ ion bound at a His₆ site, showing octahedral coordination by six histidines. (C) Expanded view of the His₆ site, with Zn²⁺ fully enclosed by six histidines drawn from both S100A8 (blue) and S100A9 (cyan), including His103 and His105 from the flexible C-terminal tail of S100A9. Credit: Randy Perera
Zinc sits at the heart of many bacterial enzymes, so one of our immune system's simplest defenses is to keep zinc out of reach. Calprotectin, a neutrophil protein abundant at infection sites, is central to this strategy. It binds transition metals with very high affinity and helps starve microbes. What we lacked was a direct, structural picture of zinc bound to calprotectin—a picture that could explain how this protein withholds zinc so effectively and what that means for pathogen biology.
In our study, we determined the first zinc-bound crystal structures of calprotectin. At the His₆ site, zinc sits in an octahedral cage coordinated by six histidines drawn from the two subunits (S100A8 and S100A9), including two from the flexible C-terminal tail of S100A9.
At the canonical His₃Asp site, zinc adopts the expected four-coordinate geometry. These structures provide the first direct evidence that calprotectin can secure Zn²⁺ with all six histidines at the His₆ site—settling a question that biochemistry had suggested but never visualized.
Structure alone does not tell you how tightly the metal is held, so we quantified zinc affinity across variants. The key result is that calprotectin keeps picomolar zinc affinity even when tail histidines are replaced or the tail is removed. The tails participate in completing the octahedral coordination we see in the structure, but high affinity does not collapse without them.
That distinction matters: coordination geometry explains how the cage closes; the affinity data explain why zinc withholding remains robust even when the tail is altered.
We then asked what these features mean for a clinically important pathogen, Staphylococcus aureus. Calprotectin strongly restricts S. aureus growth; disabling both metal-binding sites largely erases that effect, consistent with metal sequestration at the core of antimicrobial activity. The paper is in the journal Protein Science.
Looking beyond total growth to culture organization revealed more: altering or removing the S100A9 tail changed patterns of biomass accrual and composition, and it reduced the change in calprotectin–bacterium association we see with the unmodified protein. The tail therefore influences how calprotectin engages bacterial communities and how those communities assemble, even when zinc affinity remains extremely high.
Two conclusions follow. First, the new structures clarify calprotectin's chemistry: zinc is padlocked in an octahedral, six-histidine cage at the His₆ site, and zinc at the His₃Asp site is tetracoordinate. Second, calprotectin's biology is more than metal withholding. Tail-dependent interactions shape growth inhibition patterns, biomass composition, and protein–bacterium association.
Calprotectin thus acts through at least two linked layers: a chemical layer (extremely tight zinc sequestration) and an interaction layer (tail-mediated effects on how bacteria grow and adhere).
Why does this matter? S. aureus biofilms complicate wound care, device infections, and treatment failure because adherent communities are hard to eradicate. A clearer understanding of how an innate immune protein both withholds zinc and modulates biomass offers new angles for intervention.
On the design side, histidine-rich cages that become ordered upon binding offer a template for immobilizing zinc. On the biological side, preserving or enhancing tail-dependent contacts could complement antibiotics by biasing cultures away from adherent, DNA-rich biomass.
For readers who follow calprotectin, the advances are concrete: first Zn-bound crystal structures of calprotectin; direct structural evidence that Zn²⁺ is coordinated by all six histidines at the His₆ site; zinc geometry at the His₃Asp site; picomolar zinc affinity that persists without tail histidines; and a link between tail integrity and S. aureus biomass and calprotectin–bacterium association.
Together, these results sharpen the molecular picture of zinc withholding and uncover a tail-dependent dimension to how innate immunity pressures bacterial communities.
This story is part of , where researchers can report findings from their published research articles. for information about Science X Dialog and how to participate.
More information: Yasiru R. Perera et al, The C‐terminal extension of calprotectin mediates zinc chelation and modulates Staphylococcus aureus biomass accumulation, Protein Science (2025).
Yasiru Randika (Randy) Perera, Ph.D., is a structural biologist who completed his postdoctoral research at Vanderbilt University, where he investigated how metal-binding proteins shape host–pathogen interactions. His research interests include nutritional immunity, antimicrobial mechanisms, structural biology, and protein engineering.