Biocatalytic shortcut gives GLP-1-like peptides a makeover, boosting stability and potential for new therapies

Lisa Lock
scientific editor

Robert Egan
associate editor

GLP-1–pathway agonists such as semaglutide and newer multi-agonists have transformed care for obesity and diabetes, yet developers still wrestle with durability, tissue targeting, and signal "bias." Macrocyclization, tying part of a peptide into a ring, can shield drugs from degradation and favor bioactive shapes, but conventional chemistry can be costly and hard to apply late in development.
A research team at Sethera Therapeutics and the Bandarian Lab at the University of Utah has shown that a radical enzyme can "tie off" therapeutic peptides into compact rings without the usual leader-sequence requirements.
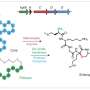
A newly published in the journalACS Bio & Med Chem Au reports a biocatalytic shortcut: an enzyme that stitches a precise thioether bond at peptide C-termini without the leader tags that many peptide-modifying enzymes usually require. In analytical readouts, the GLP-1-like analogs exhibited hallmark shifts indicative of ring formation after enzymatic processing.
The work centers on radical S-adenosyl-L-methionine (rSAM) maturases from the RiPP (ribosomally synthesized and post-translationally modified peptide) family. RiPP enzymes typically recognize an N-terminal "leader" sequence via an RRE (RiPP recognition element) for specific binding.
Here, the team demonstrated "leader-independent" activity: their process cleanly macrocyclized GLP-1-pathway analogs engineered with a C-terminal cross-linking motif, modifying the linear peptides under mild conditions. The enzyme did so even on substrates containing non-canonical residues common to marketed incretins, underscoring broad tolerance.
"From a bench perspective, the surprise was how far we could push the enzyme—no native leader, swapped leaders, non-canonical residues—and still see clean, single-ring products. That combination of tolerance and control makes PapB feel like a practical tool, not just a cool mechanism," says Jake Pedigo, lead author of the paper.
Enzymology-wise, the work straddles an unusual line, mechanistically specific yet strikingly substrate-promiscuous. While most RiPP maturases need leader/RRE interactions, the Sethera and University of Utah group modified a chimeric substrate bearing an unrelated leader and still showed activity when the RRE domain was deleted, indicating that neither canonical leader binding nor the intact RRE is strictly required.
This minimal constraint appears to reduce to a local Cys–Xⁿ–Asp/Glu motif at the cyclization site, enabling "plug-and-play" macrocycle installation with little re-engineering.
For therapeutics, the C-terminal ring can do more than tie off the chain. By rigidifying the tail, it may enhance receptor affinity or bias signaling; by capping the C-terminus, it can block proteases; and because the enzymatic process accepts diverse sequences, the ring itself can be designed as a modular "handle" to engage albumin, transporters, or disease-related receptors—routes to longer half-life, tissue targeting, or selective activity. Together, these data suggest a general, late-stage biocatalytic pathway to next-generation incretins and other peptide drugs.
"Big-pharma's GLP-1 backbones are already excellent; what we're adding is a clean, late-stage enzymatic step that can 'tie off' the C-terminus and make those molecules work even harder. By installing a precise thioether ring without leader tags, we can tune half-life, stability, and even receptor-signaling bias or tissue targeting, while staying compatible with the non-canonical residues used in today's incretins.
"In practical terms, that means faster iteration on proven scaffolds and a clearer path to differentiated, next-generation GLP-1 and multi-agonist medicines," says Karsten Eastman, CEO, Sethera Therapeutics.
More information: Jacob K. Pedigo et al, Leader-Independent C-Terminal Modification by a Radical S-Adenosyl-l-methionine Maturase Enables Macrocyclic GLP-1-Like Peptides, ACS Bio & Med Chem Au (2025). .
Provided by Sethera Therapeutics