Chemical networks can mimic nervous systems to power movement in soft materials

Stephanie Baum
scientific editor

Robert Egan
associate editor

What if a soft material could move on its own, guided not by electronics or motors, but by the kind of rudimentary chemical signaling that powers the simplest organisms? Researchers at the University of Pittsburgh Swanson School of Engineering have modeled just that—a synthetic system that on its own directly transforms chemical reactions into mechanical motion, without the need for the complex biochemical machinery present in our bodies.
Just like jellyfish, some of the simplest organisms do not have a centralized brain or nervous system. Instead, they have a "nerve net" which consists of dispersed nerve cells that are interconnected by active junctions, which emit and receive chemical signals. Even without a central "processor," the chemical signals spontaneously travel through the net and trigger the autonomous motion needed for organisms' survival.
In a study in PNAS Nexus, Oleg E. Shklyaev, research assistant, and Anna C. Balazs, Distinguished Professor of Chemical and Petroleum Engineering and the John A. Swanson Chair of Engineering, have developed computer simulations to design a soft material with a "nerve net" that links chemical and mechanical networks in a way that mimics how the earliest and simplest living systems coordinate motion.
"In living organisms, chemical signals trigger motion all the time, from the beating of heart tissue to a plant bending toward sunlight," said Balazs. "We asked, what is the simplest possible system that could reproduce this behavior in synthetic materials?"
From chemical waves to movement
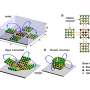
At the heart of the model is a simple feedback loop (the repressilator), which produces rhythmic chemical oscillations. The researchers replicated this system as a series of enzyme-coated microscopic beads connected by flexible links that constitute the body of the material. The beads can be viewed as mechanically-responsive tissue or vertebrae strung together into a soft spine.
When chemical reactions occur on the bead surfaces, they create waves of changing concentration that ripple along the chain. These chemical waves induce fluid motion, which in turn deforms the network, effectively converting chemistry into mechanical movement. The team calls this coupling a chemo-mechanical network (CMN).
On a visual level, Shklyaev likens the behavior to "a centipede or flatworm, where waves of contraction move through the body, propelling it forward."
The researchers found that by adjusting the chemical makeup and geometry of the network—such as arranging beads into rings—they could control the wave's length and speed. Closed loops allow motion to flow continuously around the system.
A Slinky that moves itself
Balazs offered a Slinky toy as another analogy.
"If you place a slinky at the top of the stairs and give it a simple nudge, gravity takes over and its potential energy becomes kinetic motion," she said. "Now imagine painting certain coils with enzymes that trigger specific chemical reactions. Once you start the chemistry, the Slinky moves itself, because the reactions send waves through the coils, bending and flexing them in a specific sequence of directed motion."
In this analogy, the Slinky represents the mechanical backbone, and the colored enzyme sites act like chemical nerve endings. When one site reacts, it sends a chemical "message" to its neighbors—just as neurons transmit signals—causing parts of the structure to move selectively.
"Our system can 'instruct' specific regions to move," Balazs explained. "For example, one reaction might make a section lift, while another causes a different region to flex. It's an autonomic system stripped to its simplest form—chemistry guiding mechanics."
While stimuli-responsive materials can harness external stimuli to produce a given move, the materials are typically receptive to only one or two distinct cues and undergo a limited repertoire of motion. Here, the coated beads produce position-specific and enzyme-specific chemical signals and thus, altering the chemistry and positions of the coated beads can give rise to a broad range of dynamic behavior.
A chemical nervous system
The team's model demonstrates how chemical reaction networks (CRNs) can give rise to mechanical coordination without any electronics or centralized control.
"This simple system doesn't need a brain or an electrical signal," said Shklyaev. "Once the chemical reaction starts, it generates flows that make the structure move and is completely self-contained."
The work reveals an inherent connection between CRNs operating in the body fluid and the submerged elastic tissues (beads and links) that leads to the formation of a corresponding chemo-mechanical network (CMN). The human body is 60% water and replete with enzymes. Through various mechanisms, enzymatic reactions in aqueous solutions intrinsically generate gradients, which can translate chemical energy into mechanical action.
The formation of CMNs and processes describing the interactions between CRNs and elastic tissues are often neglected in biology. The CRN–CMNs creates a closed chemical circuit that sends and receives signals, generates motion, and can even transport microscopic cargo along its structure, like how biological tissues move nutrients or respond to stimuli.
The concept could inform future soft robots, responsive materials, or chemical computing systems that operate autonomously in fluid environments.
Simple components, complex behavior
"Biology shows us that complexity emerges from simplicity," Balazs said. "By combining only a few key components—chemistry, elasticity, and fluid flow—we see a material move. It converts chemical fuel into motion, coordinates its parts, and performs work without needing wires, circuits, or motors.
"It's a bit like eating a cheeseburger, and then moving your arm," she joked. "You add fuel, and it does the rest."
Ultimately, the research provides a blueprint for building autonomous, adaptive materials—soft systems that think in chemistry instead of electricity.
More information: Oleg E Shklyaev et al, Chemical signaling in reaction networks generates corresponding mechanical impulses, PNAS Nexus (2025).
Journal information: PNAS Nexus
Provided by University of Pittsburgh