Cluster of norovirus virions. Credit: CDC
Human noroviruses, GII.4 strains in particular, are the chief drivers of acute viral gastroenteritis around the world, a condition for which there are no vaccines or antivirals. Understanding how these viruses enter cells in the gut, a first step toward developing an infection, can lead to effective therapeutics.
With that goal in mind, researchers at Baylor College of Medicine and collaborating institutions investigated the entry mechanism of GII.4 human noroviruses, comparing the dominant strain GII.4 Sydney with other GII.4 variants.
The study, published in the , reveals that human GII.4 noroviruses have evolved a uniquely potent entry mechanism with clear strain-specific differences. The findings open new possibilities for identifying the elusive human norovirus receptor as well as developing vaccines and treatments.
"To study the entry mechanism of human norovirus GII.4, we compared the binding and entry of multiple GII.4 variants using GII.4 virus-like particles (VLPs) and human intestinal enteroids," said first author of the work Dr. B. Vijayalakshmi Ayyar, senior staff scientist in the Department of Molecular Virology and Microbiology at Baylor.
VLPs are non-infectious, protein structures that mimic the shape and size of viruses but lack the viral genetic material, making them incapable of replicating or causing disease. Human intestinal enteroids are a laboratory model of the human gastrointestinal tract that recapitulates its cellular complexity, diversity and physiology. Human enteroids mimic strain-specific host-virus infection patterns, making them an ideal system to dissect human norovirus infection, identify strain-specific growth requirements and develop and test treatments and vaccines.
"In a , we discovered that the binding of human norovirus GII.4 VLPs to enteroid cells wounds the cells' membranes, which in turn triggers a membrane repair mechanism to the injury site, activating another cellular pathway known as the CLIC pathway," Ayyar said. "We observed crosstalk between CLIC-mediated internalization of viral particles and host repair mechanisms."
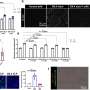
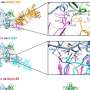
In this study, the researchers studied the entry mechanism in more detail. "We found that after viral particles bind to human enteroids, they form clusters on the cell surface triggering a series of events that result in viral entry and infection," said co-author, Dr. B. V. Venkataram Prasad, professor of molecular virology and microbiology and Alvin Romansky Chair in Biochemistry at Baylor. "Importantly, our study revealed that structure changes in the viral particles are required to assemble the dynamic clusters on the cell surface, driving a multistep entry pathway."
Interestingly, the researchers discovered that not all GII.4 variants form distinct clusters on the cell surface. Clustering strains, which include GII.4 Sydney, cause significantly more membrane wounding and enter and replicate in enteroids more than non-clustering strains.
"When we studied differences between clustering and non-clustering GII.4 strains, we identified two amino acids on the protruding domain of the norovirus particles, named V333 and R339, that were critical mediators of clustering and entry," Ayyar said. "Mutating or blocking these amino acids disrupted clustering and viral entry."
"We know there are many differences among different human norovirus strains. Some of them relate to the immunology of the virus and some to how the virus enters the cell," said co-author Dr. Robert L. Atmar, John S. Dunn clinical research professor of medicine—infectious diseases and of molecular virology and microbiology at Baylor. "Learning more about what distinguishes individual strains so we can better understand why some viruses predominate more than others is exciting. This study on GII.4 human norovirus is an important step toward that goal."
"By increasing our understanding of how human norovirus enters susceptible cells, these findings are bringing us closer to identifying and characterizing the elusive host receptor for human norovirus," said corresponding author Dr. Mary K. Estes, Distinguished Service Professor and Cullen Foundation Endowed Chair of molecular virology and microbiology at Baylor.
"This work also contributes important insights that advance the development of targeted preventive treatments and therapies that we hope one day will contribute to relieve the burden these viruses pose to the human population."
Other contributors to this work include Carmen V. Apostol, Janam Jitendra Dave, Soni Kaundal, Joseph A. Kendra, Frederick H. Neill, Khalil Ettayebi, Sarah Maher, Ramakrishnan Anish, Gabriel I. Parra, Göran Larson and Sue E. Crawford. The authors are affiliated with Baylor College of Medicine, Food and Drug Administration or the University of Gothenburg in Sweden.
More information: B. Vijayalakshmi Ayyar et al, Functional diversity in GII.4 norovirus entry: HBGA binding and capsid clustering dynamics, Proceedings of the National Academy of Sciences (2025).
Journal information: Proceedings of the National Academy of Sciences
Provided by Baylor College of Medicine