A 'stick-peel-reuse' adhesive based on lock-and-key chemistry

Lisa Lock
scientific editor

Robert Egan
associate editor

If you have ever felt the frustration of trying to re-stick a used sticky note, you will understand the challenge of reversible adhesion. Adhesives that can strongly bond to surfaces, be peeled off, and then reused are in high demand for industrial applications.
Unfortunately, the strong bonds formed by conventional adhesives are permanent, such that these adhesives cannot be reused. But now, in a study in Advanced Materials, researchers at The University of Osaka report the invention of a new polymeric adhesive that can be reused repeatedly.
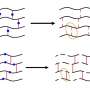
When two materials come into contact with each other, a region (called an interface) forms between them that contains molecules of both materials. When the interface is wide, it is difficult to pull the materials apart and they are considered to adhere strongly to each other. Adhesion between materials can be activated and deactivated by introducing reversible bonds into this interface.
Reversible bonds are bonds that break and reform under specific conditions. Host–guest complexation is one way of creating reversible bonds. A host is typically a large molecule with a cavity into which a smaller guest molecule can fit, analogous to a lock and a key. The guest molecule seated in the host forms a host–guest complex.
"Guest and host molecules need to be able to move toward each other for these complexes to form, but polymer molecules are bulky and cannot move easily," explains lead author Kenji Yamaoka. "Thus, complex formation at polymer–polymer interfaces is inefficient, making it difficult to engineer reversible adhesion in polymer systems."
At a special temperature called the glass-transition temperature (Tg), segments of polymer chains go from being in a frozen glass-like state to moving freely. The higher the polymer temperature is above Tg, the more easily the segments can move.
The research team fabricated two polymers that can reversibly bond with each other. The researchers adjusted Tg to help the polymers move freely toward each other. Then, to fully understand the mechanism behind reversible adhesion, the team deflected neutrons off the interface to visualize how the sticking and peeling occurred at a molecular level.
"We found that controlling the temperature or adding/removing chemicals enables complexes to break and reform, resulting in peeling and re-adhesion on demand," says Yoshinori Takashima, senior author. "Our findings are exciting because this would be useful for many industries."
The novel adhesive can be decomposed on demand and reused multiple times, which could improve the manufacturing yield of precision devices, reduce costs, and minimize waste. The team's research will no doubt be of interest to manufacturers, but also will aid waste reduction and recycling.
More information: Kenji Yamaoka et al, Supramolecular Interface Engineering via Interdiffusion for Reusable and Dismantlable Polymer Adhesion, Advanced Materials (2025).
Journal information: Advanced Materials
Provided by University of Osaka