Shanghai Tower serves as inspiration for first synthetic dynamic helical polymer

Gaby Clark
scientific editor

Robert Egan
associate editor

Researchers at the University of Groningen in the Netherlands have developed a polymer that adopts a coiled spring configuration at low temperatures and unfolds again upon heating. Furthermore, the molecule can break down into smaller molecules under certain conditions. The Shanghai Tower, with its spiral shape, served as the inspiration for the project following a visit five years ago. A description of the resulting helical polymer was recently in Nature Chemistry.
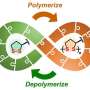
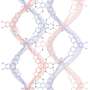
Spiraling structures are common in biological molecules. A well-known example is the double helix of DNA; another is the alpha-helix domains in proteins. Various artificial helices have been created, some of which can change their shape. Other synthetic polymers can be recycled into their monomers, but so far, no polymers have been developed that can both change shape and be recycled into their chemical building blocks.
Broken down in living tissues
Qi Zhang, postdoc at the University of Groningen, and colleagues have designed and synthesized such a molecule, based on amino acids and disulfides. At low temperatures, the molecule looks like a coiled spring, which unfolds upon heating. "These helical polymers could be suitable for biomaterials," explains Zhang. "For example, they might interact with cell membranes or proteins."
In certain circumstances, the molecule can deconstruct itself, mirroring the way proteins are fragmented into amino acids. In contrast to synthetic plastics, the building blocks of these molecules can be broken down in living tissues. However, such applications are still far off, as the current generation only works in organic solvents. "There is still a lot to be explored," says Zhang.
The team was led by University of Groningen organic chemist Ben Feringa, one of the recipients of the 2016 Nobel Prize in Chemistry. Zhang recounts that the initial design for the molecule was sketched by Prof. Feringa on a napkin in the Shanghai Tower, which had just inspired the two of them to discuss helices. From that moment on, it took about five years and the collaboration of six institutes in three countries to turn the sketch into reality. "We all enjoyed exchanging knowledge and experience in this project."
More information: Qi Zhang et al, Dual dynamic helical poly(disulfide)s with conformational adaptivity and configurational recyclability, Nature Chemistry (2025).
Journal information: Nature Chemistry
Provided by University of Groningen