Ultrafast photothermal process achieves 3,000°C in 0.02 seconds, boosting hydrogen production efficiency sixfold

Lisa Lock
scientific editor

Robert Egan
associate editor

The rapid and energy-efficient synthesis of high-performance catalysts is a critical hurdle in advancing clean energy technologies like hydrogen production. Addressing this challenge, a research team at KAIST has now developed a novel platform technology that utilizes a 0.02-second flash of light to generate an ultrahigh temperature of 3,000°C, enabling the highly efficient synthesis of catalysts.
This breakthrough process reduces energy consumption by more than a thousandfold compared to conventional methods while increasing hydrogen production efficiency by up to six times, marking a significant step toward the commercialization of clean energy.
A joint research team co-led by Professor Il-Doo Kim from the Department of Materials Science and Engineering and Professor Sung-Yool Choi from the School of Electrical Engineering developed the direct-contact photothermal annealing platform. This technique synthesizes high-performance nanomaterials through brief exposure to intense light, generating a transient temperature of 3,000°C in just 0.02 seconds.
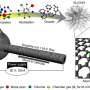
Using this intense photothermal energy, the researchers successfully converted chemically inert nanodiamond (ND) precursors into highly conductive and catalytically active carbon nanoonions (CNOs).
More impressively, the method simultaneously functionalizes the surface of the newly formed CNOs with single atoms. This integrated, one-step process restructures the support material and embeds catalytic functionality in a single light pulse, representing a significant innovation in catalyst synthesis. The research is in the journal ACS Nano.
CNOs, composed of concentric graphitic shells, are ideal catalyst supports due to their high conductivity, large specific surface area, and chemical stability. However, traditional CNO synthesis has been hindered by complex, multi-step post-processing required to load metal catalysts and by reliance on energy-intensive, time-consuming thermal treatments that limit scalability.
To overcome these limitations, the team leveraged the photothermal effect. They devised a method of mixing ND precursors with light-absorbing carbon black (CB) and applying an intense pulse from a xenon lamp. This approach triggers the transformation of NDs into CNOs in just 0.02 seconds, a phenomenon validated by molecular dynamics simulations.
A key innovation of this platform is the simultaneous synthesis of CNOs and functionalization of single-atom catalysts (SACs). When metal precursors, such as platinum (Pt), are included in the mixture, they decompose and anchor onto the surface of the nascent CNOs as individual atoms. The subsequent rapid cooling prevents atomic aggregation, resulting in a perfectly integrated one-step process for both synthesis and functionalization.
The team has successfully synthesized eight different high-density SACs, including platinum (Pt), cobalt (Co), and nickel (Ni). The resulting Pt-CNO demonstrated a sixfold enhancement in hydrogen evolution efficiency compared to conventional catalysts, achieving high performance with significantly smaller quantities of precious metals. This highlights the technology's potential for scalable and sustainable hydrogen production.
"We have developed, for the first time, a direct-contact photothermal annealing process that reaches 3,000°C in under 0.02 seconds," said Professor Il-Doo Kim. "This ultrafast synthesis and single-atom functionalization platform reduces energy consumption by more than a thousandfold compared to traditional methods. We expect it to accelerate the commercialization of technologies in hydrogen energy, gas sensing, and environmental catalysis."
More information: Dogyeong Jeon et al, Photothermal Annealing-Enabled Millisecond Synthesis of Carbon Nanoonions and Simultaneous Single-Atom Functionalization, ACS Nano (2025).
Journal information: ACS Nano