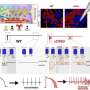
Calmodulin (CaM) C-lobe binding regulates TRPA1 activity. Credit: Nature Communications (2025). DOI: 10.1038/s41467-025-63767-7
A new Yale study has uncovered the hidden mechanical move that may regulate pain signaling in the human body—and why it sometimes goes awry.
Since launching her own lab at Yale in 2018, Candie Paulsen and her research team have been working to crack a longstanding mystery: How are pain signals regulated in the human body? And why do they sometimes go haywire?
While researchers had long known that calcium plays a key role in alerting the body to pain, they didn't understand how the response turns off or why the interaction sometimes goes awry, leading to chronic pain and inflammation.
For years they'd focused on TRPA1, a protein channel in nerve cells that lets calcium flow in, allowing the body to sense pain and trigger inflammation. Too much calcium can damage cells. Somewhere along the TRPA1 channel, researchers deduced, was a mechanism that shut down the calcium flow—and the resulting pain signals—but no one could pin it down.
"We finally found it," said Paulsen, an assistant professor of Molecular Biophysics and Biochemistry at the Wu Tsai Institute at Yale who led an all-Yale research team at her Science Hill lab. The hidden culprit, they report in a new published in Nature Communications, is a protein called calmodulin, which Paulsen's team discovered plays a key role in turning off the TRPA1 channel after calcium has passed through it.
How? By latching onto a docking site at the far end of the channel. This latching is essential for shutting down the activated channel before it becomes overactive, which can fuel pain and inflammation.
The findings, Paulsen said, may lead to better interventions in pain management.
Paulsen's seven-member research team included Yale graduate students, a postdoctoral researcher, and a postgraduate researcher. The lead author is Justin Sanders, who recently began a position as an industrial postdoctoral fellow at Helix BioStructures, a pharmaceutical laboratory in Indianapolis. Sanders initiated this research as the Paulsen lab's first graduate student.
"We figured out exactly what calmodulin is doing and where it's binding," Paulsen said. "It was a really proud mentoring moment for me because we've been working on this for a long time, chasing it down, trying to figure out what's going on."
Shortly before Paulsen came to Yale, a team of U.K. researchers identified calmodulin as an important regulator of the TRPA1 channel, but they predicted a different attachment point to the channel.
Paulsen recalled Sanders' disappointment when he uncovered that the U.K. study's predicted site had fallen short. "I remember us having a conversation, and I said, "Justin, this just means there's a discovery out there that hasn't been made. This is that moment in science when you realize you're on the cusp of figuring out something.'"
To reach that next step, Paulsen's team first altered TRPA1 so that calmodulin was unable to attach anywhere in the channel and then stayed open too long and became hyperactive (a possible cause of persistent pain signaling in people.)
To peer deeper into the channel's workings, the team used a mix of laboratory techniques, including protein interaction tests to determine where calmodulin might be attaching; imaging calcium to see how it behaves in human cells; and molecular modeling to see how calmodulin fit onto the newly discovered docking site.
While Sanders moved onto a postdoctoral research position at the University of Georgia in June 2024, his work in the lab brought researchers one step closer to unraveling the mystery of chronic pain, Paulsen said.
"Identifying this binding site where attachment is critical to turn off the channel properly really clued us in," she said. "And there could be any number of things happening that lower the ability of calmodulin to bind at that site, which could dysregulate the channel and support the transition to chronic pain, for sure. Cracking that is the next step."
More information: Justin H. Sanders et al, Calmodulin binding is required for calcium mediated TRPA1 desensitization, Nature Communications (2025).
Journal information: Nature Communications
Provided by Yale University