Nanotechnology innovation may revolutionize gene detection in a single cell
Scientists at Arizona State University’s Biodesign Institute have developed the world’s first gene detection platform made up entirely from self-assembled DNA nanostructures. The results, appearing in the January 11 issue of the journal Science, could have broad implications for gene chip technology and may also revolutionize the way in which gene expression is analyzed in a single cell.
“We are starting with the most well-known structure in biology, DNA, and applying it as a nano-scale building material, ” said Hao Yan, a member of the institute’s Center for Single Molecule Biophysics and an assistant professor of chemistry and biochemistry in the College of Liberal and Sciences.
Yan is a researcher in the fast-moving field known as structural DNA nanotechnology — that assembles the molecule of life into a variety of nanostructures with a broad range of applications from human health to nanoelectronics.
Yan led an interdisciplinary ASU team to develop a way to use structural DNA nanotechnology to target the chemical messengers of genes, called RNA.
The team included: lead author and chemistry and biochemistry graduate student Yonggang Ke; assistant professor of chemistry and biochemistry Yan Liu; Center for Single Molecule Biophysics director and physics professor Stuart Lindsay; and associate professor in the School of Life Sciences, Yung Chang.
"This is one of the first practical applications of a powerful technology, that, till now, has mainly been the subject of research demonstrations,” said Lindsay. “The field of structural DNA nanotechnology has recently seen much exciting progress from constructing geometrical and topological nanostructures through tile based DNA self-assembly initially demonstrated by Ned Seeman, Erik Winfree and colleagues,” said Yan.
A recent breakthrough of making spatially addressable DNA nanoarrays came from Paul Rothemund’s work on scaffolded DNA origami, a method in which a long, single-stranded viral DNA scaffold can be folded and stapled by a large number of short synthetic “helper strands” into nanostructures that display complex patterns.
“But the potential of structural DNA nanotechnology in biological applications has been underestimated, and if we look at the process of DNA self-assembly, you will be amazed that trillions of DNA nanostructures can form simultaneously in a solution of few microliters, and very importantly, they are biocompatible and water soluble,” said Yan.
DNA chip and microarray technology have become a multi-billion dollar industry as scientists use it to examine thousands of genes at the same time for mutations or uncovering clues to disease. However, because DNA probes are pinned to the solid surface of the microarray chips, it is relatively slow process for the targets to search and find the probes. Also, it is hard to control the distances between the probes with nanometer accuracy.
“In this work, we developed a water soluble nanoarray that can take advantage of the DNA self-assembling process and also have benefits that the macroscopic DNA microchip arrays do not have,” said Yan. “The arrays themselves are reagents, instead of solid surface chips.”
To make the DNA origami RNA probes, Yan has taken advantage of the basic DNA pairing rules in the DNA chemical alphabet (“A” can only form a zipper-like chemical bond with “T” and “G” only pair with “C”). By controlling the exact position and location of the chemical bases within a synthetic replica of DNA, Yan programmed a single stranded genomic DNA, M13, into nanotiles to contain the probes for specific gene expression targets.
Yan refers to the self-assembled DNA nanoarrays as nucleic acid probe tiles, which look like a nanosized postage stamp. In a single step, the M13 scaffold system can churn out as many as 100 trillion of the tiles with close to100 percent yield.
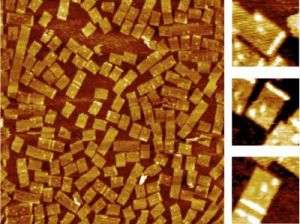
Yan’s team designed three different DNA probe tiles to detect three different RNA genes along with a bar code index to tell the tiles apart from each other. “Each probe can be distinguished by its own bar code, so we mixed them together in one solution and we used this for multiplex detection,” said Yan. The group uses a powerful instrument, atomic force microscopy (AFM), which allows the researchers to image the tiles at the single molecule level.
On the surface of each DNA probe tile is a dangling single stranded piece of DNA that can bind to the RNA target of interest. “Each probe actually contains two half probes, so when the target RNA comes in, it will hybridize to the half probes and turn the single stranded dangling probes into a stiff structure,” said Yan. “When it is stiffened, it will be sensed by the atomic force microscope cantilever, and you can see a bright line, which is a height increase. The result is a mechanical, label-free detection.”
The technology is able to detect minute quantities of RNA. “Since the DNA-RNA hybridization has such a strong affinity, in principle, a single molecule would be able to hybridize to the probe tile,” said Yan.
Although there are still many technical hurdles yet to overcome, the group is excited about the potential applications of the technology. “What our approach provides is that the probe tiles are a water-soluble reagent, so the sample volume can potentially be shrunk down to the volume of a single cell level. Our ultimate goal is to detect RNA gene expression at the single cell level.”
Source: Arizona State University




















