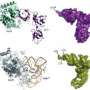
Molecular model of the Spy protein. Spyโs cradle shape may be the key to its ability to help fold and protect other proteins. (Courtesy of S. Quan, University of Michigan/HHMI).
(ย้ถนา๙ิบOrg.com) -- James Bond frequently has to undertake spectacular feats to protect Queen and country against utter destruction under insurmountable odds. But what happens when the homeland is a bacterial cell, and the danger comes from the insurmountable odds of making large amounts of a complex molecule? You call in a sleeper agent โ a protein called Spy.
Thatโs the discovery made by biochemist James Bardwell and his team from the University of Michigan, Howard Hughes Medical Institute, McGill University and the National Research Council of Canadaโs Biotechnology Research Institute. Their findings, which included a model of the protein obtained from the Canadian Light Source, were published in the journal Nature Structural and Molecular Biology.
Many proteins used in pharmaceuticals, such as insulin, can be manufactured by bacteria that have had the instructions for making the desired protein inserted into their genetic code. However, the process doesnโt work well for all proteins, leading to botched batches of poorly-folded molecular clumps that are unable to function properly. Bardwell and his colleagues wanted to see if the system could be improved by making the bacteria an offer that was hard to refuse โ linking protein production to their survival.
โWe gave the bacteria a pretty stark choice โ fold proteins or die,โ explained Dr. Bardwell. โWe linked making a stable target protein in strains of E. coli to their resistance to penicillin, and then grew them in a medium containing the drug. The bacteria could survive only if they could also fold the target protein really well.โ
The result: surviving bacteria produced up to 700 times more of the desired target protein than they would normally, along with large amounts of a little protein called Spy (short for spheroplast protein Y). It turns out Spy is a molecular chaperone โ a type of molecule that facilitates the folding of proteins. The amount of Spy normally present in cells is vanishingly small, but in response to stress it is made in huge amounts . It is particularly induced in response to threats that would cause proteins inside the bacterium to unravel or clump uselessly, such as alcohol or tannins. While Spy had been previously identified inside bacteria, its function wasnโt understood until it was implicated in these โfold or dieโ experiments.
โA lot of people were surprised that such well-studied bacteria as E. coli had a chaperone that had remained undiscovered,โ said Dr. Bardwell. โBut many assay tests that look for chaperones wouldnโt find Spy; then again, no one else has tried to genetically select for protein folding like we did.โ
To better understand the workings of their enigmatic chaperone, the team needed to determine Spyโs molecular structure. Dr. Bardwell turned to his collaborator, Prof. Mirek Cygler at McGill University and the NRC Biotechnology Research Institute, who in turn sent crystals of Spy to the CLS.
The molecular model obtained from the CLS data revealed that Spy had even more surprises in store. Spy is one of the thinnest chaperone molecules ever found โ a cradle-shaped molecule consisting of two parts, only nine ten-millionths of a millimetre thick (the same thickness, roughly, of nine hydrogen atoms) or about one fortieth the size of some better known chaperones. Spyโs cradle-shape may allow it to surround larger proteins, enabling them to fold properly while protecting them from harmful influences.
โIt kind of acts like Teflon or a candy wrapper, covering the proteins and keeping them from clumping and sticking together,โ Dr. Bardwell noted.
Now that Spy has been identified, Dr. Bardwell and his team plan to study how the protein works in detail, while also looking for similar chaperones using the same genetic selection routine. They also hope to force bacteria to produce other poorly folding, unstable proteins like HIVโs โtail protein,โ seen by many as the target for a potential AIDS vaccine.
โSpy is the way E. coli fights off stressful environments by protecting proteins from unfolding,โ he explained. โBy making Spy fold the proteins we want, there are lots of places we could go.โ
More information: Quan et al. 2011. Genetic selection designed to stabilize proteins uncovers a chaperone called Spy. Nature Stuctural and Molecular Biology
Provided by Canadian Light Source