August 7, 2019 feature
A hybrid material that switches reversibly between two stable solid states
Solid matter typically contains a single, stable solid state for a specific set of conditions. Materials scientists envision that new materials with interchangeable solid states will be advantageous for diverse technical applications. In a new report now published on Nature Materials, Fut (Kuo) Yang and colleagues in the interdisciplinary departments of Chemical Engineering, Bioengineering and Biotechnology in Canada and China described the development of a two-in-one hybrid material.
They composed the material with a polymer impregnated with a supercooled salt solution known as "sal-gel." The material assumed two distinct but stable and reversible solid states under varying temperatures (-90 degrees C to 58 degrees C) and pressures. When the scientists stimulated nucleation, the material shifted from a clear, soft solid to a white hard state that was 104 times stiffer than the original (15 kPa vs. 385 MPa). They reversed the hard solid back to soft consistency via transient heating to demonstrate reversibility of the transition. The study explored the concept of robust physical metastability of a liquid state and Yang et al. extended the work to sugar alcohols to form stimuli-responsive and non-evaporating "sug-gel." Such two-in-one hybrid materials will be useful in soft robotics and adhesive applications.
offer a solution to engineer paradoxical shape adaptability and load-bearing capabilities that are important for a variety of technical areas including , and . Sustaining the mechanical response of such smart materials is, however, limited by the requirement for an external stimulus. A solution to create two-in-one solids is to explore the mechanical or structural metastability of such materials. This is observed with metamaterials that can transform their stiffness via changes in topological states.
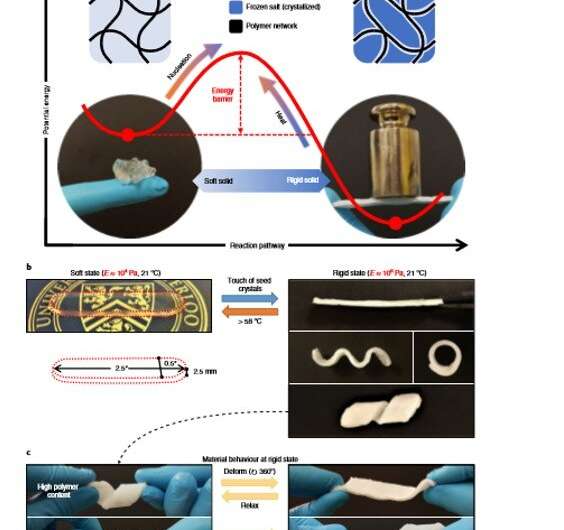
To obtain dual stable states, the underlying mechanism must form an energy barrier between the two, where each state rested at an energetic minimum. For instance, liquid crystallization can meet this requirement where the liquid initially required formation of a sufficiently large cluster of crystalline atoms or molecules. The free energy gain of transforming the crystalline phase must then overcome the free energy cost of creating an interface between the . Scientists could overcome the energy barrier between the interfaces by inducing self-assembly via (formation of new crystals from existing crystals) for liquid-to-crystalline transformations and with heat for crystalline-to-liquid transformation. The process is comparatively more difficult for phase transformations with pure solids whose crystalline and non-crystalline phases are both solids.

As an example material, is a phase-changing material (PCM) commonly known as 'hot ice," as it releases heat during freezing with well-known capacity to supercool. Although the solid has a melting point of 58 degrees C, it can remain stable as a supercooled liquid at room temperature for years, with application in . Yang et al. provided the solid sodium acetate trihydrate an additional solid form by using a compatible polymer network to produce a printable and light-weight hybrid material known as sal-gel. The material could interchangeably switch its effective stiffness without external stimulation, allowing the scientists to fully leverage the phase transition and metastability of the salt.
The hybrid material transformed to a rubber-like form on melting for on-demand shape-fixing with a change in stiffness greater than 104 times. The feature is highly desirable for "two-in-one" solids compared to previously developed . The new material is relevant for the ever-growing performance to miniaturize and increase performance density of multifunctional materials.

The scientists prepared sal-gel by mixing melted sodium acetate trihydrate with polymer pre-cursors of and a liquid mixture of acetic acid with water. The resulting gel mixture remained transparent, suggesting the constituents to be miscible with each other. The resulting gel had two solid states; a transparent soft state and an opaque rigid state that resisted deformation. The scientists transformed the sal-gel from its soft state to the rigid state via secondary nucleation through the touch of sodium acetate trihydrate seed crystals. Upon contact with a seed crystal, nucleation occurred immediately for crystallization to proceed from the point-of-contact across the entire material.
To conduct the initiation experimentally, Yang et al. used a wooden stick with a small amount of fine crystal dust at the tip. Since the phenomenon originated from the sample surface, they assumed a two-fold cause; where at first, the free energy cost for nucleation greatly lowered at the gel's surface due to reduced surface area. Thereafter, on contact, the surface experienced an enormous . As long as the gel remained lubricated, the scientists could prevent unwanted crystallization. Yang et al. reversed the sal-gel to its soft state by heating it above the melting point and used these properties to fix the shape of the gel on-demand. They tuned the physical properties of the frozen state by manipulating the polymer content of the gel to withstand deformation and return to its fixed shape upon stress release.
The research team tested the mechanical behavior of the two states of the sal-gel system under similar environments using . They compared the melted and frozen sal-gel, where a visible plastic deformation formed on the frozen state, which disappeared after melting. Using measurements Yang et al. showed a significant change in stiffness between the two states. Although the frozen sal-gel was stiff, it was less brittle for indentation without cracking compared to a polymer-free frozen salt control.
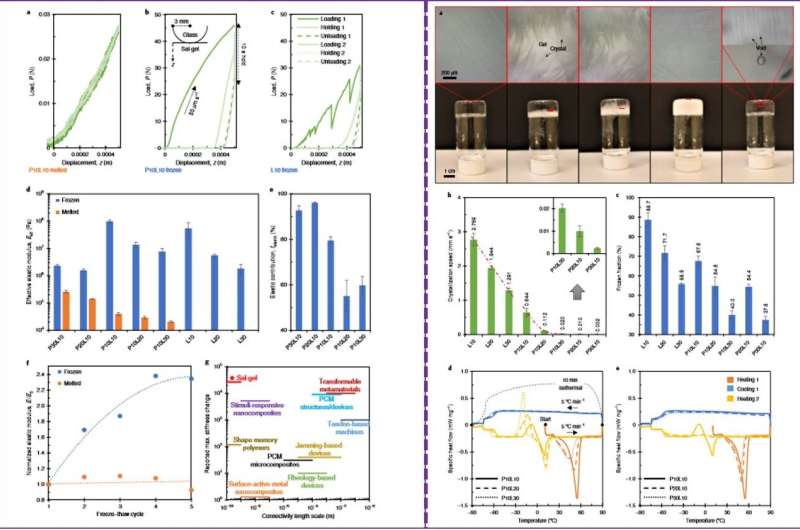
After further characterization of the hybrid material, the scientists showed that when more acetic acid liquid was present in the mixture, the sal-gel became softer and less elastic. When they repeatedly froze and thawed the gel, they did not observe lasting damage in the polymer network, although the material became stiffer with repeated freeze-thaw cycles.
The scientists then investigated the crystallization behavior of the sal-gel and observed the growing crystals to push aside the polymer network without rupturing or damaging the network. The salt hydrate demonstrated thermal behaviors similar to , where the addition of more polymer and diluent led to less crystallization. The thermal behaviors indicated a strong stability of sal-gel with supercooling greater than 150 degrees C.
The sal-gel demonstrated soft-to-hard transitional contact, instant and robust self-adhesion, mechanical energy storage alongside the capacity to form smart constructs. A major advantage of the hybrid material was its self-contained nature, which easily allowed additive manufacture. As a proof-of-principle, Yang et al. fabricated a synthetic sea cucumber using three-dimensional doodling by delivering un-crosslinked sal-gel solution using a syringe to crosslink the solution in the lab using an ultraviolet light source thereafter. The resulting print closely resembled a living sea cucumber in appearance and mechanics, where the dermis switched between a transparent soft and opaque rigid state.
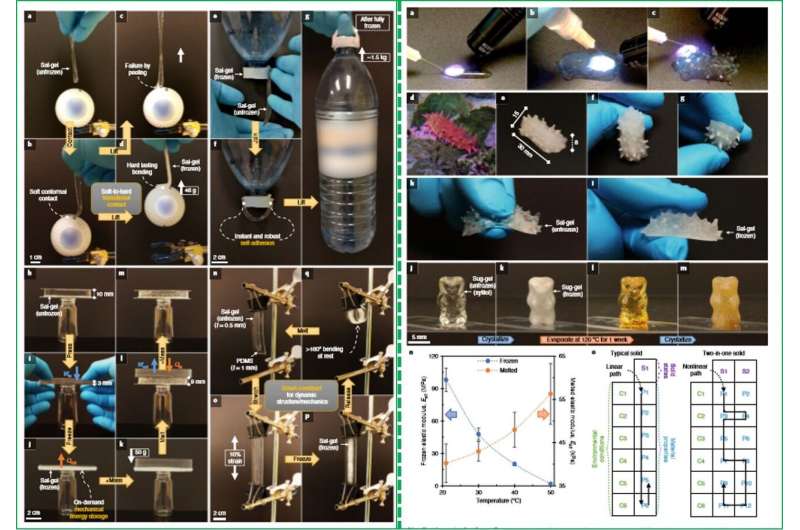
They extended the concept of sal-gel to other materials using the sugar alcohol as the PCM (phase-changing material). Using the sugar alcohol, they prepared a sugar-gel (sug-gel) gummy bear with two-solid state behaviors. When the scientists overheated the construct to 120 degrees C to accelerate evaporation for a week, the gummy bear's volume did not visibly change and still remained capable of dual-state behavior.
To translate sal-gel into practical applications, Yang et al. should resolve two technical issues relative to evaporation and sensitivity, which affected the implementation of the material. The issues were partially addressed by coating the sal-gel with lubricant to optimize and increase its stability, they aim to further engineer the material in the future and fully resolve the limitation. The research team also increased the flexibility in design and functionality in the two-in-one solid, compared to normal solids with a single solid state.
In this way, Fut (Kuo) Yang and co-workers strategically constructed a solid framework inside a functional liquid—supercooled melted salt (sodium acetate trihydrate) by forming a compatible polymer network of poly(acrylic acid) to create the hybrid material sal-gel. Synergistic interactions of the materials at the molecular level allowed Yang et al. to leverage the liquid properties and explore its phase transition and metastability.
The hybrid construct showed unusual material behavior to switch between two stable solid states with varying mechanical properties that could co-exist under similar environmental conditions. The stiffness states did not require continuous stimulation, allowing new capabilities for advanced applications. While the present work focused on the transformation of supercooled liquid, Yang et al. expect to extend the approach to other liquids with different functionalities to diversify the range of mechanically switchable materials.
More information: Fut Yang et al. A hybrid material that reversibly switches between two stable solid states, Nature Materials (2019).
J. R. Capadona et al. Stimuli-Responsive Polymer Nanocomposites Inspired by the Sea Cucumber Dermis, Science (2008).
Aditya Balasubramanian et al. Shape-Memory Microfluidics, Advanced Functional Materials (2013).
Journal information: Nature Materials , Science , Advanced Functional Materials
Provided by Science X Network
© 2019 Science X Network