November 25, 2019 feature
Determining topographical radiation dose profiles using gel nanosensors

Thamarasee Jeewandara
contributing writer
The routine measurement of radiation doses can be clinically challenging due to limitations with conventional used to measure the dose uptake of external ionizing radiation. In a new study, Karthik Pushpavanam and an interdisciplinary team of researchers in the departments of Chemical Engineering, Molecular Sciences, Banner MD Anderson Cancer Center and Arizona Veterinary Oncology in the U.S. has described a novel gel-based nanosensor. The technology allows and quantification of topographical radiation dose profiles during radiotherapy.
Upon exposure to ionizing radiation, the scientists converted gold ions in the gel in to gold nanoparticles (AuNPs) accompanied with a visual change in gel color due to properties. They used the intensity of color formed in the gel as a quantitative reporter for ionizing radiation and first used the gel nanosensor to detect complex topographical dose patterns after administration to models followed by applications with live canine patients undergoing clinical radiotherapy. The ease of fabrication, operation, rapid readout, colorimetric detection and relatively low cost of the technology implied for topographical dose mapping during clinical radiotherapy applications. The research work is now published on Science Advances.
Advances in radiation therapy have led to and state-of-the-art planning software to deliver high conformal radiation doses to patients for . During radiotherapy, a high dose is typically delivered to a target tumor while . During patients are administered with larger in order to conclude treatment within a short time frame. However, during such procedures can lead to overdosing and .
To minimize accidental overexposure, researchers seek to independently at or near the target tissue for advanced patient safety. Technically, both molecular and nanosensors can overcome limits present in conventional systems to form practical alternatives as . However, their should be addressed and alleviated to develop robust and effective sensors that quantitatively and qualitatively determine the topographical dose profiles during clinical radiotherapy.

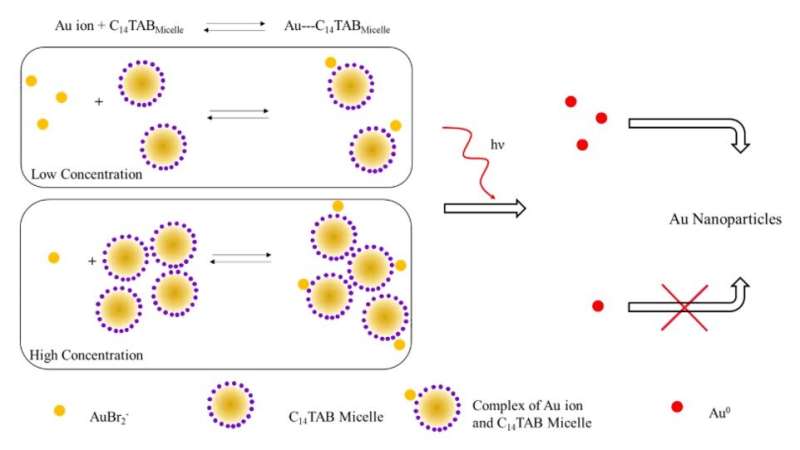
Since gold nanoparticles (AuNPs) have unique physical and chemical characteristics that provide an excellent platform to develop sensors. Pushpavanam et al. engineered a colorimetric sensor where ionizing radiation caused AuNP formation from . The formation of a gel-based nanosensor can allow easy handling and applications during .
In the present work, the team demonstrated colorimetric detection and quantification of dose distribution profiles using a gel nanosensor to topographically map radiation doses along tissue surfaces. During preclinical evaluations, the team administered the gel nanosensor technology in live canine patients undergoing radiotherapy. In total, the results indicated the scope of the technology for clinical translation in human patients and the capacity to determine topographic doses to plan treatments and verify dosages during cancer radiotherapy.
During the experiments, the conversion of gold ions into nanoparticles was accompanied by a maroon color development in the irradiated region of the gel nanosensor. While gold exists in a trivalent state in general (AuCl4-) it can be reduced to a metastable +1 valence state (AuBr2-) at room temperature . The irradiation of gels containing therapeutic levels of radiation stimulated or the splitting of water molecules into . The radiolysis-generated hydrated electrons in turn reduced monovalent gold to form gold atoms in its (Au0) that nucleated and matured into maroon colored AuNPs. The intensity varied with the dose of radiation and the team used the range of linear responses to calibrate the gel nanosensor. Based on this principle, Pushpavanam et al. determined the response of the fully irradiated gels to calibrate absorbance with radiation dose.
To determine intensity of the color and dose delivered within gels after irradiation, the researchers used and observed a decrease in the spectral profile width, with increasing radiation dose for decreased (ratio of the percentage of the standard deviation to the average value) of the nanoparticles. The peak absorbance intensity increased with increasing radiation dose to corroborate the observed increase in color intensity.
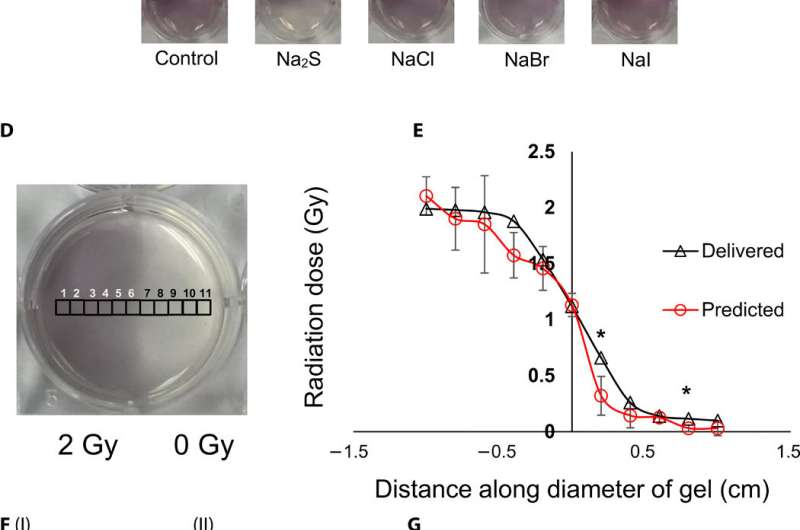
To understand the gel nanosensor's ability to detect topographical distribution of the radiation dose, the scientists irradiated half of the gel nanosensor with a 4 gray (Gy) dose. The maroon-color only appeared in the irradiated area confirming AuNP formation, but after one hour of exposure, the color bled into the irradiated region showing loss of topographic information in the gel with time. The team observed the phenomenon to arise from reaction-controlled conditions and not based on the gel composition. By incubating the gel with sodium sulfide (Na2S) for 10 minutes, they suppressed the color bleed-over and reasoned that to the ability to quench unreacted gold ions in the nonirradiated region and preserve dose information accurately for dose visualization and dosimetry. The scientists adopted the sensor for wide dose ranges by modulating the time of Na2S addition; to achieve a level of flexibility hitherto unavailable in clinical dose detection systems.
The research team then used the gel nanosensor to visualize diverse topographical radiation patterns, where the intensity of the color increased with increasing dose while preserving topographical integrity. As proof of concept, they showed the gel nanosensor's ability to detect complex radiation patterns with a model dose patterned to form "ASU" (after Arizona State University). Then using (TEM), the scientists characterized the generated gold nanoparticles as a function of dose to observe reduced average nanoparticle diameter and polydispersity at higher doses of radiation. They followed this with (EDS) to detect higher yields of AuNPs in the irradiated regions of the gel nanosensor as expected.
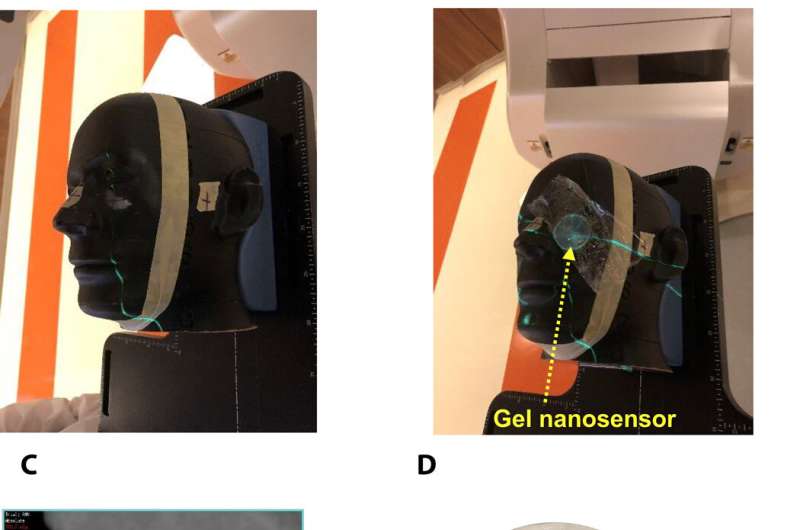
To investigate translational potential of the gel nanosensor and predict topographical profiles of radiation, Pushpavanam et al. first used a head and neck phantom model. They delivered an irregular crescent-shaped radiation dose near the eye to mimic clinically challenging administration modes of radiotherapy close to critical structures such as the eye during skin cancer treatment. The dose profile delivered using the treatment planning system was in excellent agreement with the predictions of the gel nanosensor. Indicating its capability to detect and predict complex radiation patterns similar to those used in clinical human radiotherapy.
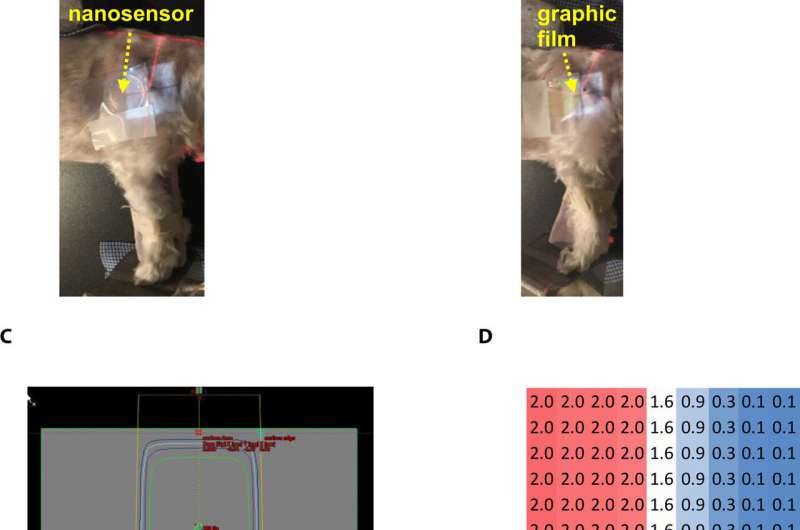
During preclinical studies, the research team used two canine models undergoing radiotherapy to investigate the efficiency of gel nanosensors as independent, nanoscale radiation dosimeters for the first time and compared the efficiency with conventional clinical . On completion of the treatment, Pushpavanam et al. observed maroon color formation in one-half of the gel, whereas the non-irradiated region remained colorless. They showed predictions of the gel nanosensor in the irradiated region to agree excellently with the treatment planning system and the radiochromic film. The gel nanosensor also predicted for the region external to the irradiation to receive minimal radiation and their topographical dose profiles as well. The performance was comparable to clinical radiochromic films but with faster than conventional wait times (typically >24 hours) to obtain the results. The scientists demonstrated the simplicity of fabrication, operation, readout time and cost effectiveness ($ 0.50 per gel material only) of the . They maintained the response of the gel nanosensor for at least seven days to indicate long-term retrieval of dosing data unlike with fluorescence-based dosimeters with readouts that lasts mere minutes.
In this way, Karthik Pushpavanam and colleagues developed the first colorimetric gel nanosensor as a nanoscale dosimeter to detect and distinguish regions exposed to irradiation. They optimized the platform with a chemical quenching agent (Na2S) to accurately reveal topographical dose distribution during clinical radiotherapy. The scientists can control the pore size distribution of the gel substrate to enhance efficacy of the nanosensor. They tested the efficiency of the gel nanosensor to predict complex topographical dose profiles in anthromorphic head and neck phantoms and in live canine patients undergoing radiotherapy. The highly disruptive and translational potential of the gel nanosensor technology will lead to improved patient safety and outcomes in clinical radiotherapy.
Written for you by our author —this article is the result of careful human work. We rely on readers like you to keep independent science journalism alive. If this reporting matters to you, please consider a (especially monthly). You'll get an ad-free account as a thank-you.
More information: Karthik Pushpavanam et al. Determination of topographical radiation dose profiles using gel nanosensors, Science Advances (2019).
Karthik Pushpavanam et al. A Colorimetric Plasmonic Nanosensor for Dosimetry of Therapeutic Levels of Ionizing Radiation, ACS Nano (2015).
Nashrulhaq Tagiling et al. Effect of scanning parameters on dose-response of radiochromic films irradiated with photon and electron beams, Heliyon (2018).
Journal information: Science Advances , ACS Nano
© 2019 Science X Network
















